CiPA logoThe International Council on Harmonization (ICH) S7B and E14 regulatory guidelines were introduced in 2005 to evaluate the proarrhythmic liability of new drugs.
Selective permeation of potassium ions (K+) through voltage-dependent potassium (Kv) channels has an important role in setting the resting membrane potential of cells and repolarising the action potential. The voltage-gated potassium channel family is composed of forty members and they are separated into twelve classes Kv1 to Kv12.
The major α pore forming subunits of Kv channels are composed of 6 membrane spanning domains (S1-6) that contain a pore (P) loop located between the S5 and S6 domains (Figure 1A). These subunits interact to form functional tetrameric channels (Figure 1B). The P loop makes up the ion conduction pathway of the channels and consists of approximately 20 amino acids. The subunits are arranged in such a fashion that the S5-P-S6 subunits face one another (Figure 1B), forming the walls of the central water filled pore. The Kvchannels possess a glycine-tyrosine-glycine (GYG) signature motif within the ion conduction pathway, which is thought to confer K+ ion selectivity. Mutation to the GYG sequence results in a loss of K+ selectivity. The voltage sensitivity of the channels has been attributed to the S4 domains, which contain a large number of positively charged amino acid residues, which respond to a change in membrane potential.
A number of Kv channels (e.g. some of the Kv1 and Kv7 families) are capable of generating functional heterotetramers between family members, which can affect the electrophysiological characteristics and pharmacology cf. the homotetrameric channel. You can learn more about previous work on Kv1.x tetramers as drug discovery targets in this publication.
(https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/cmdc.201100600)
Heterotetramerisation can be an important consideration if a structure-based drug discovery approach is adopted, as understanding the topology of the different subunits enables the generation of optimal in silico models.
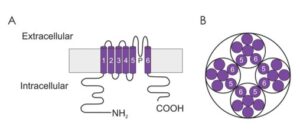
Figure 1: Putative membrane topology of the voltage-gated potassium channel α subunit.
The electrophysiological properties of Kv channels can vary significantly, even within members of the same family. For example, the rate of inactivation of the Kv1.x channels can vary substantially (Figure 2).
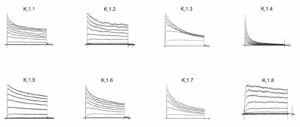
Figure 2: Representative current traces from Metrion’s Kv1.x panel.
Metrion is highly experienced at developing custom Kv1.x (e.g. Kv1.3, Kv1.5) screening assays to identify state-dependent inhibitors. Metrion has developed screening assays suitable for testing several therapeutic modalities (small molecules, peptides and other biologics) against Kv1.x ion channels on the manual patch-clamp platform and various automated patch-clamp platforms (e.g. QPatch 48). Most assays can be configured to detect state-dependent modulation and deliver information-rich data during the primary structure-activity screening assay (Figure 3). Metrion typically test compounds using a cumulative four-point concentration-response paradigm (Figure 3), but can amend the testing paradigm to reflect clients’ requirements. For example, for slow acting agents such as antibodies or peptides, it might be preferable to use a single-point testing format to enable an accurate determination of block (Figure 4).
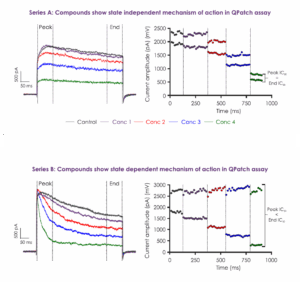
Figure 3: Representative current traces demonstrating that state-dependent inhibitors of Kv1.x channels can be identified using a cumulative four-point concentration-response assay paradigm.

Figure 4: Representative data from a biologic screened using a single-point assay paradigm.
Contact us to discuss ion channel drug discovery research.
CiPA logoThe International Council on Harmonization (ICH) S7B and E14 regulatory guidelines were introduced in 2005 to evaluate the proarrhythmic liability of new drugs.
Ion channels are large multi-subunit transmembrane proteins that control the flow of charged ions across the cell membrane, leading to changes in cellular excitability and intracellular signalling.