'Time Is a Critical Factor When Evaluating Oligonucleotide Therapeutics in Human Ether-a-Go-Go-Related Gene Assays’ (Nucleic Acid Therapeutics) receives ‘2024 Technology Innovation Publication Award’.
High-quality cardiac safety screening services, including GLP hERG and CiPA assays
The cardiac safety screening assays we offer include:
Assessment of core cardiac ion channels: hERG, NaV1.5 and CaV1.2
Expanded CiPA cardiac panel: hERG, NaV1.5 (late current), CaV1.2, Kir2.1, KV4.3_KChIP and KV7.1_MinK
Additional cardiac channels: HCN4 and KV1.5
Read about CiPA screening and hERG screening
GLP hERG profiling to support Investigational New Drug (IND) applications
Performed in accordance with current ICH S7B guidelines
Read about GLP hERG testing
High throughput/high resolution assessment of compound effects on action potential morphology using voltage dye
Predicts free compound exposure associated with 10ms change in clinical QTc following both acute (30 min)and chronic (24 h) treatment
Defines probability of clinical QRS liability
Aligns with current ICH S7B guidelines
Assess proarrhythmic potential of compounds via manual current clamp
Read about hiPSC-derived cardiomyocyte screening
Functionally assess the viability following chronic compound treatment (up to 72 h) using high-throughput impedance platform
By working with us you benefit from:
Cardiac safety team led by expert with 25 years experience in drug discovery and in vitro safety pharmacology within the pharmaceutical industry - see our Leadership Team.
Exceptional ion channel electrophysiology and drug discovery expertise.
A team of experienced cell biologists to create novel cell lines.
High quality, cost-effective compound screening.
Detailed characterisation of lead compounds in a range of high-quality assays.
Translational services including confirmation of efficacy in stem cell and other phenotypic models.
Flexible approach that best suits your project and budget.
Rapid turn-around times, reporting and data interpretation by highly experienced ion channel scientists.
Our expertise in GLP hERG screening is demonstrated in the case study GLP hERG Assay Validation Following ICH E14/S7B 2022 Q&A Best Practice Guidelines.
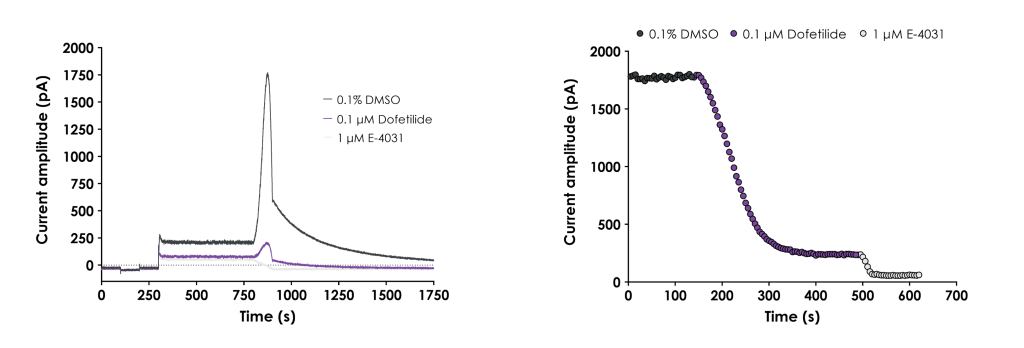
Top: Example traces in vehicle, dofetilide and E-4031. Bottom: Corresponding IT plot. IC50 values generated were within 2-fold of the values reported in the ICH E14/S7B training material. Read more about GLP hERG assay validation.
Download the recording of this webinar to learn how an hiPSC-CM model can help provide clear decision-making data for your project team that can avoid costly issues related to QTc and QRS cardiac liabilities in the clinic.
Recording includes presentations and Q&A:
Derek Leishman (VP Translational and Quantitative Toxicology, Eli Lilly and Company).
Steve Jenkinson (VP Drug Discovery and Safety, Metrion).
'Time Is a Critical Factor When Evaluating Oligonucleotide Therapeutics in Human Ether-a-Go-Go-Related Gene Assays’ (Nucleic Acid Therapeutics) receives ‘2024 Technology Innovation Publication Award’.
Students learned about electrophysiology concepts, sodium channels in drug discovery and the commercial business-side of ion channels