Validation of chronic cardiotoxicity assays
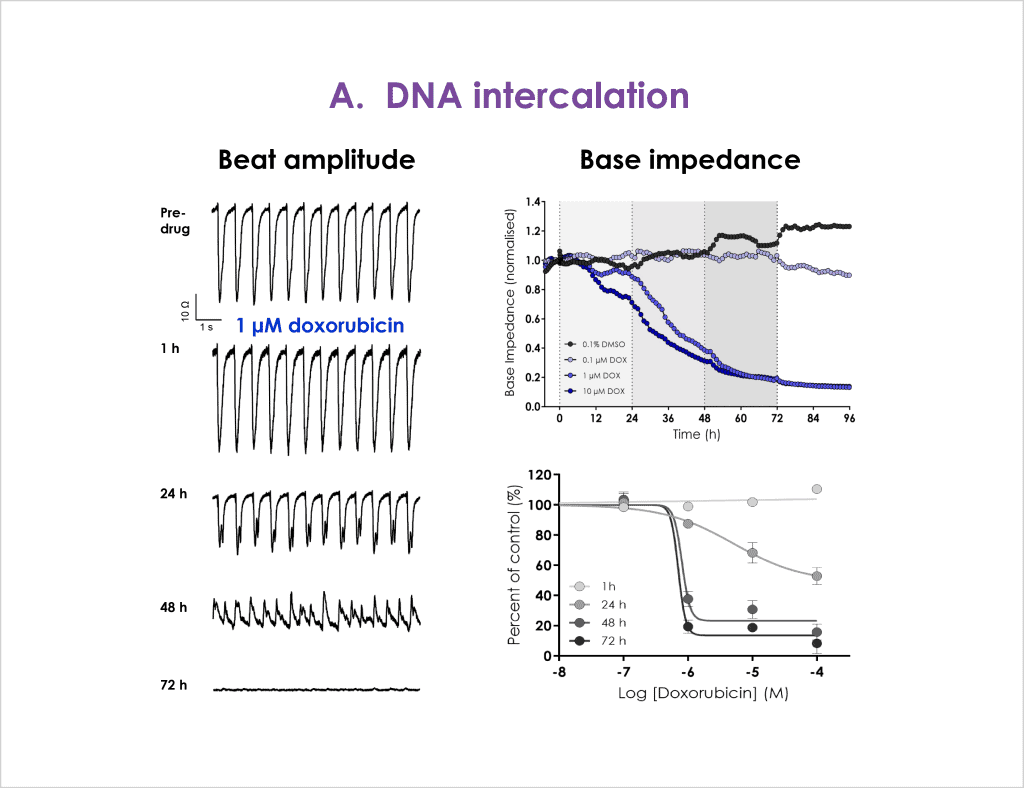
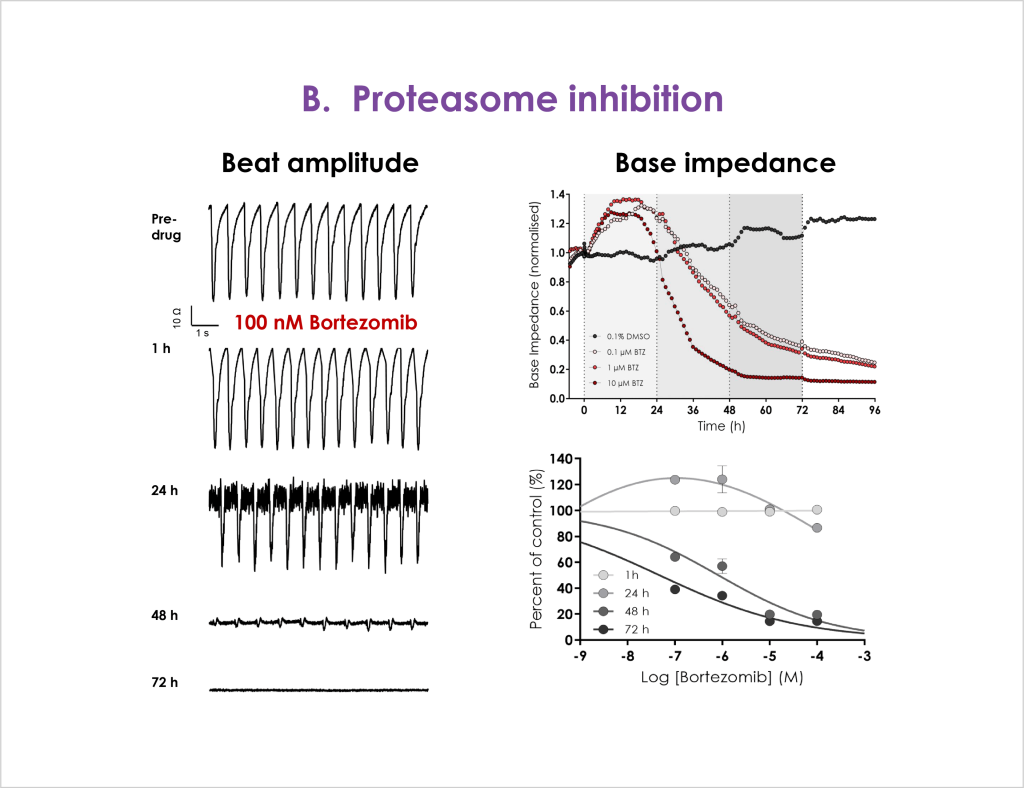
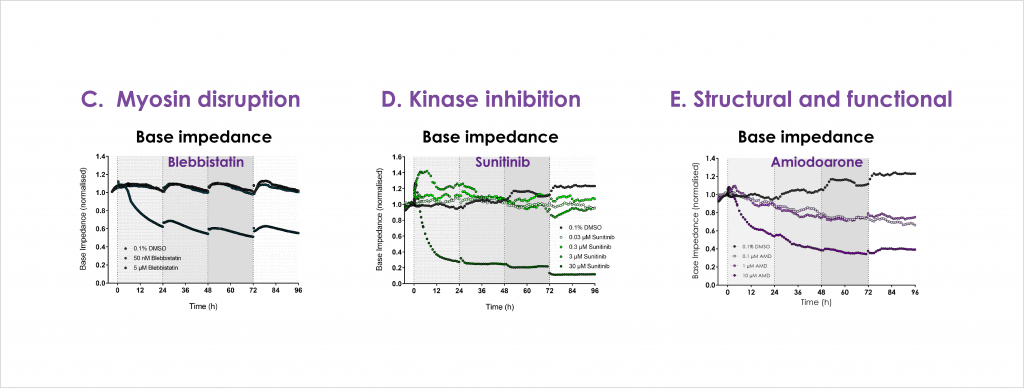
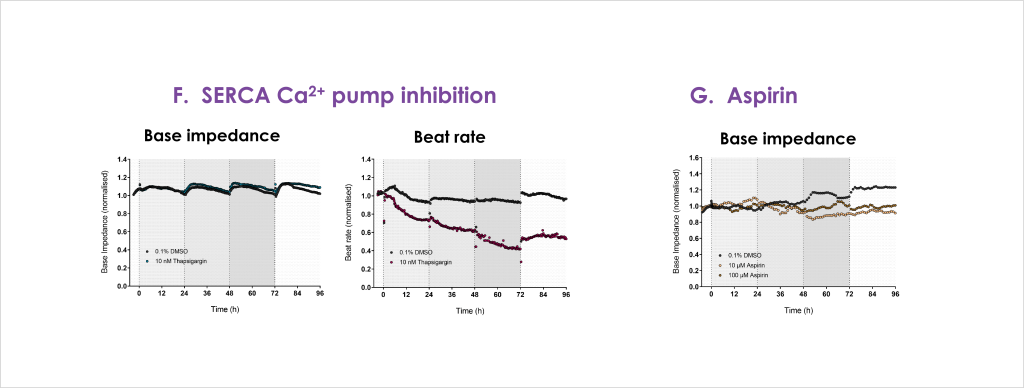
Cardiac toxicity remains the leading cause of new drug safety side-effects. Current preclinical cardiac safety assays rely on in vitro cell-based ion channel assays and ex vivo and in vivo animal models⁽¹⁾. These assays provide an indication of acute risk but they do not always predict the effect of chronic compound exposure, as recently seen with oncology drugs⁽²⁾. Therefore, new assays are required to characterise chronic structural and functional effects in human cells earlier in drug discovery⁽²̛ ³⁾. Impedance-based technology can provide more accurate chronic cardiotoxicity measurements in an efficient manner using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs)⁽¹̛ ³⁾.
Here we outline validation of chronic cardiotoxicity assays using an impedance platform in combination with commercial hiPSC-CMs:
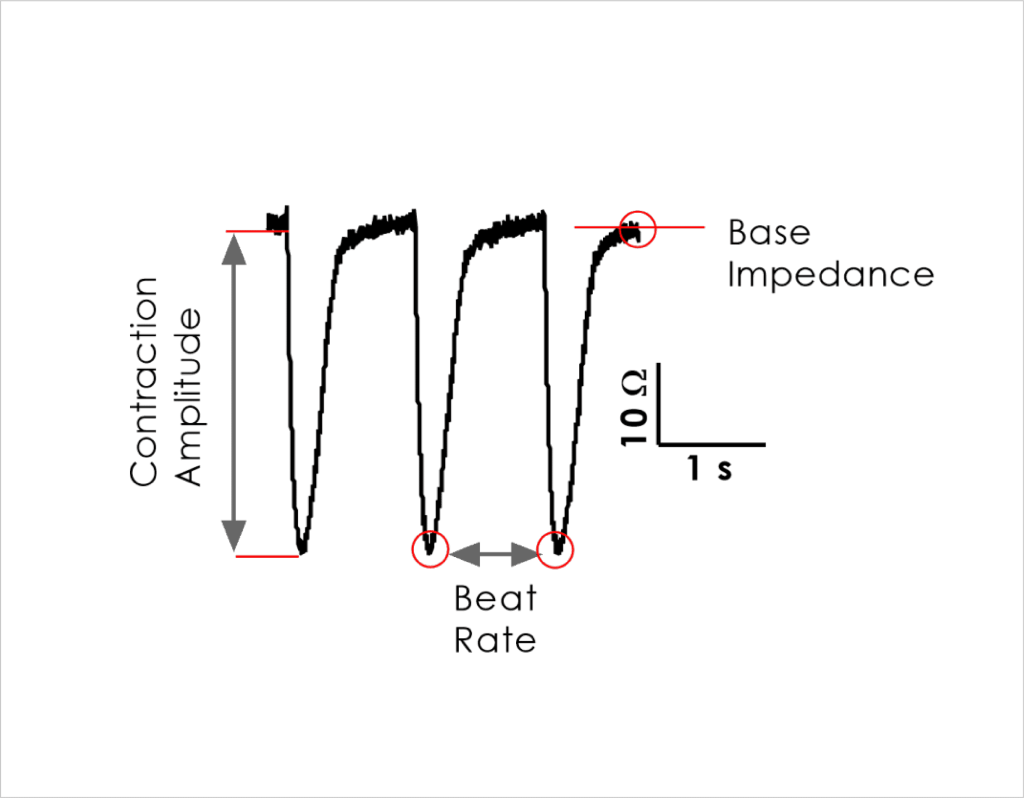
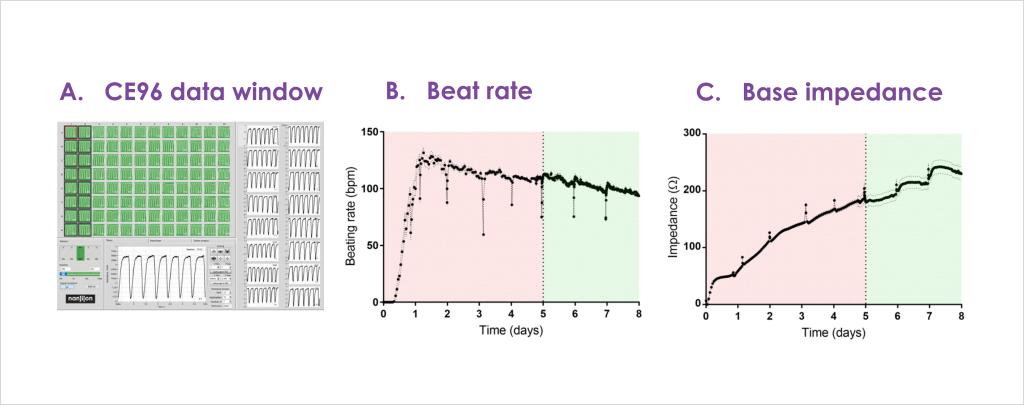
- Impedance readouts can be reliably measured and are stable to allow acute (< 24 hrs) as well as longer duration (72 hrs) testing.
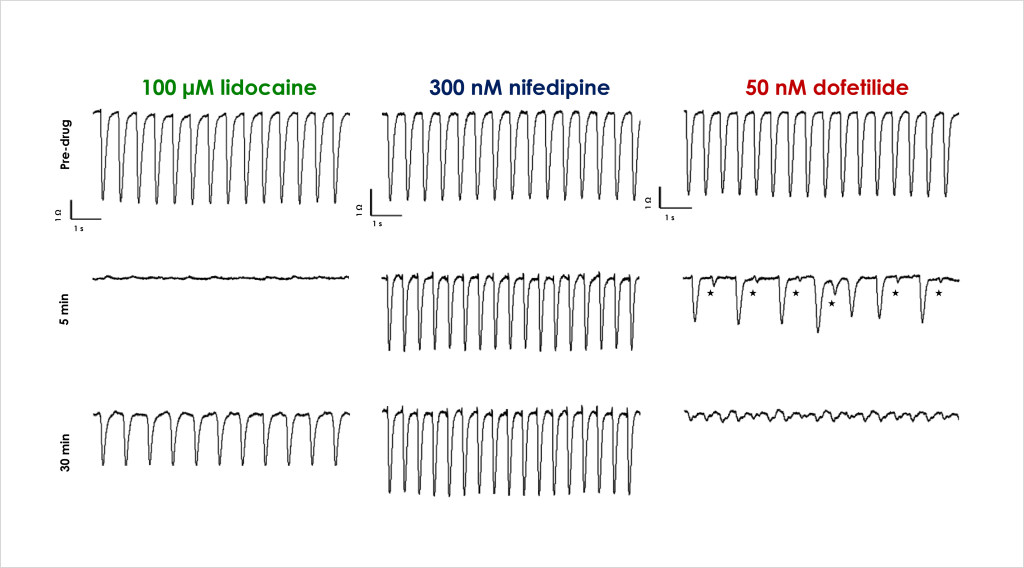
- Identify the acute and chronic effects of clinical cardiac ion channel modulators against cardiomyocyte contractility and viability.
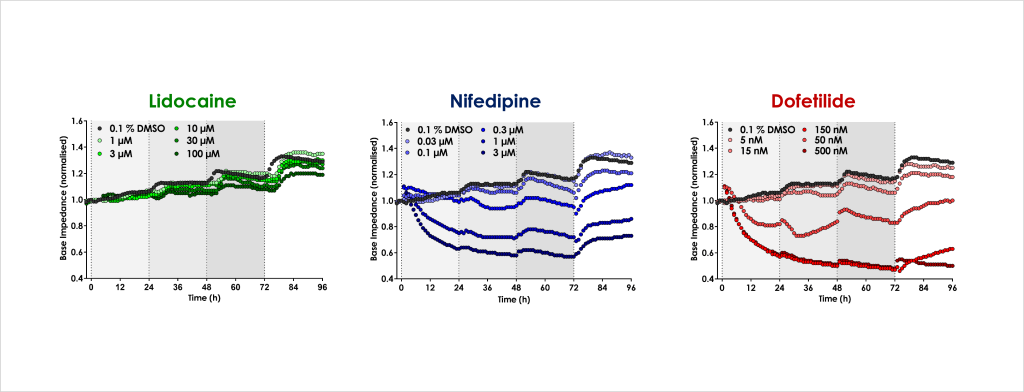
- Confirm that structural and functional cardiotoxicity of clinical compounds arising from multiple cellular and signalling mechanisms could be detected in an acute and/or chronic assay timeframe.