CiPA logoThe International Council on Harmonization (ICH) S7B and E14 regulatory guidelines were introduced in 2005 to evaluate the proarrhythmic liability of new drugs.
Selective permeation of sodium ions (Na+) through voltage-dependent sodium (Nav) channels is fundamental for the generation of action potentials in excitable cells such as neurones and muscles. The voltage-gated sodium channel family is composed of nine pore-forming members, which are named Nav1.1 to Nav1.9 and encoded by genes referred to as SCN1A to SCN11A.
The channels consist of an α subunit – a large membrane spanning protein that forms the ion conduction pore – and one to two accessory (β) subunits. Whilst the α subunits are capable of forming functional channels, the association of β subunits can affect their biophysical properties (e.g. voltage-dependence) and cellular localisation. The α-subunit is composed of four repeat domains, labelled I through IV, each containing six membrane-spanning segments, labelled S1 to S6. The highly conserved S4 segment acts as the channel’s voltage sensor whilst the S5-S6 loop helix incorporates the pore and the C-terminus inactivation gate.
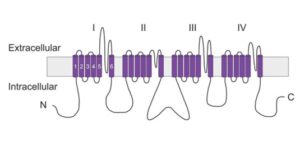
Figure 1: Putative membrane topology of the voltage-gated sodium channel α subunit.
The important role of Nav channels in controlling the activity of excitable cells is supported by their involvement in various channelopathies (Table 1). This has fuelled a strong interest in the pharmaceutical industry to modulate the activity of sodium ion channels in a variety of therapeutic indications, especially pain and epilepsy. Fully understanding the electrophysiological properties of Nav channels is pivotal in developing efficacious and safe therapeutics. For example, companies focused on developing Nav channel inhibitors have tended to focus on identifying compounds that show a preference to the inactivated channel state, as Nav channels will accumulate in the inactivated state during periods of high frequency firing associated with patho-physiology. Metrion is highly experienced at developing custom Nav1.x screening assays to identify state-dependent inhibitors. Figure 2 shows representative data using a voltage protocol (Figure 2A) designed to evaluate the state-dependent inhibition of Nav channels. Four different concentrations of Amitriptyline were applied and the inhibition was determined for the first (P1; resting state inhibition) and the third (P3; inactivated state inhibition) depolarising steps (Figure 2B). Figure 2C shows the four-point concentration-response curves constructed using the inhibition data determined for currents elicited at P1 and P3. This voltage protocol demonstrated that Amitriptyline has a preference to the inactivated state of Nav channels. Metrion has similar assay validation data available for most Nav1.x channels (e.g. Nav1.7, Nav1.8 and Nav1.5), please enquire for more information.
Contact us to discuss ion channel drug discovery research.
CiPA logoThe International Council on Harmonization (ICH) S7B and E14 regulatory guidelines were introduced in 2005 to evaluate the proarrhythmic liability of new drugs.
Ion channels are large multi-subunit transmembrane proteins that control the flow of charged ions across the cell membrane, leading to changes in cellular excitability and intracellular signalling.