Access high-value, underexploited pain targets
The global chronic pain treatment market was valued at $88.2 billion in 2023 and is projected to reach $173.4 billion by 2033, growing at a 7.0% CAGR. Neuropathic pain, a major segment of this market, represents around 31.6% of the total.1
NaV1.9 is a key ion channel target for pain drug discovery, particularly for development of novel non-opioid treatments for chronic pain. However, the path to progressing therapies targeting NaV1.9 has been blocked by lack of robust and reliable NaV1.9 high-throughput screening assays.
Metrion has overcome these obstacles with a NaV1.9 screening cascade, built upon a robust and stable hNaV1.9 cell line, using automated patch-clamp technology and translational assays. This solution enables:
- Clearer vision of growth for pain drug discovery pipeline and develop more effective treatments for chronic pain in this under explored and clinically relevant area
- High-quality reproducible data allowing rapid hit identification, hit expansion and SAR.
- Consistent and reproducible results.

Unique approach to NaV1.9 drug discovery for faster, smarter, cost-effective screening
Our NaV1.9 suite of services supported by highly experienced electrophysiologists, lead by a Leadership team with over 100 years’ experience in drug discovery research, sets the Metrion service apart from others. The business case is clear for organisations looking to rapidly meet project milestones:
- Shorter timelines: High-quality screening assays accelerate project milestones.
- Lower costs: Reliable screening data to robustly support medicinal chemistry.
- Cost-effective outsourcing: Rapid, expert data interpretation ensures confident advancement of promising compounds.
- High confidence in NaV1.9 screening cascade:
- Identify high quality hits
- Accelerate hit-to-lead discovery
- Investigate mechanism of action via translational assays
For business leaders, this translates into:
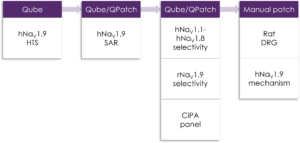
Figure 1. Example of Metrion's NaV1.9 screening cascade.
Reliable, high-throughput NaV1.9 screening for faster, consistent and accurate results
Our validated, high-throughput NaV1.9 screening solution delivers consistent and accurate results. Key advantages:
- High-throughput NaV1.9 screening for rapid compound testing: The CHO-hNaV1.9 cell line, developed in-house by Metrion, enables automated patch-clamp screening using the Qube 384 platform. This system allows rapid testing of large compound libraries and hit-to-lead support.
- Reliable NaV1.9 data, fewer setbacks: The stable and robust expression of Metrion’s NaV1.9 cell line ensures high reproducibility and low assay variability. We have demonstrated high sensitivity, robustness and accuracy of NaV1.9 screening data during the assay development with the testing of tool compounds and compound plates randomly spiked with a NaV1.9 inhibitor.
The business challenge: Missed opportunities
The NaV1.9 ion channel is a promising target for developing non-opioid chronic pain treatments, but challenges in establishing screening assays have slowed progress across the industry. Key issues include:
- Functional expression challenges: difficulty in expressing NaV1.9 in stable heterologous systems.
- Biophysical properties: activation and inactivation kinetics of NaV1.9 require voltage-control only offered by automated electrophysiological techniques.
Metrion's unique approach overcomes these challenges and unlocks the potential for increased ROI in pain drug discovery.
Choose Metrion as your CRO: Contract research services for preclinical drug discovery beyond NaV1.9
 Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
This breadth allows customers to consolidate their ion channel research needs with a single provider, streamlining project management and communication, and enabling them to benefit from:
- Industry-leading expertise: A team of highly skilled scientists with extensive knowledge of ion channel pharmacology
- State-of-the-art technology: Access to automated patch clamp systems for high-throughput screening
- Gold standard electrophysiology: Experts in all in vitro manual patch clamp configurations
- Extensive collection of ion channel cell lines: Access to broad range of ion channel targets for selectivity assessment, including CiPA panel
- Customised research solutions: Tailored services designed to meet specific project needs in both in vitro and translational assays
- Fast and reliable data reporting: Accelerating the pace of drug discovery with rapid turnaround times
- Translational assays: Validation of lead compounds in sensory neurons
- Comprehensive scientific support: Providing detailed data interpretation and expert guidance
Take the next step: Accelerate your drug discovery pipeline with Metrion
Don’t let NaV1.9 screening challenges stall your pipeline. Partner with Metrion to access reliable, high-throughput solutions designed for success - contact us today:
Reference
- https://market.us/report/chronic-pain-treatment-market/
Access high-value, underexploited pain targets
The global chronic pain treatment market was valued at $88.2 billion in 2023 and is projected to reach $173.4 billion by 2033, growing at a 7.0% CAGR. Neuropathic pain, a major segment of this market, represents around 31.6% of the total.1
NaV1.9 is a key ion channel target for pain drug discovery, particularly for development of novel non-opioid treatments for chronic pain. However, the path to progressing therapies targeting NaV1.9 has been blocked by lack of robust and reliable NaV1.9 high-throughput screening assays.
Metrion has overcome these obstacles with a NaV1.9 screening cascade, built upon a robust and stable hNaV1.9 cell line, using automated patch-clamp technology and translational assays. This solution enables:
- Clearer vision of growth for pain drug discovery pipeline and develop more effective treatments for chronic pain in this under explored and clinically relevant area
- High-quality reproducible data allowing rapid hit identification, hit expansion and SAR.
- Consistent and reproducible results.

Unique approach to NaV1.9 drug discovery for faster, smarter, cost-effective screening
Our NaV1.9 suite of services supported by highly experienced electrophysiologists, lead by a Leadership team with over 100 years’ experience in drug discovery research, sets the Metrion service apart from others. The business case is clear for organisations looking to rapidly meet project milestones:
- Shorter timelines: High-quality screening assays accelerate project milestones.
- Lower costs: Reliable screening data to robustly support medicinal chemistry.
- Cost-effective outsourcing: Rapid, expert data interpretation ensures confident advancement of promising compounds.
- High confidence in NaV1.9 screening cascade:
- Identify high quality hits
- Accelerate hit-to-lead discovery
- Investigate mechanism of action via translational assays
For business leaders, this translates into:

Figure 1. Example of Metrion's NaV1.9 screening cascade.
Reliable, high-throughput NaV1.9 screening for faster, consistent and accurate results
Our validated, high-throughput NaV1.9 screening solution delivers consistent and accurate results. Key advantages:
- High-throughput NaV1.9 screening for rapid compound testing: The CHO-hNaV1.9 cell line, developed in-house by Metrion, enables automated patch-clamp screening using the Qube 384 platform. This system allows rapid testing of large compound libraries and hit-to-lead support.
- Reliable NaV1.9 data, fewer setbacks: The stable and robust expression of Metrion’s NaV1.9 cell line ensures high reproducibility and low assay variability. We have demonstrated high sensitivity, robustness and accuracy of NaV1.9 screening data during the assay development with the testing of tool compounds and compound plates randomly spiked with a NaV1.9 inhibitor.
The business challenge: Missed opportunities
The NaV1.9 ion channel is a promising target for developing non-opioid chronic pain treatments, but challenges in establishing screening assays have slowed progress across the industry. Key issues include:
- Functional expression challenges: difficulty in expressing NaV1.9 in stable heterologous systems.
- Biophysical properties: activation and inactivation kinetics of NaV1.9 require voltage-control only offered by automated electrophysiological techniques.
Metrion's unique approach overcomes these challenges and unlocks the potential for increased ROI in pain drug discovery.
Choose Metrion as your CRO: Contract research services for preclinical drug discovery beyond NaV1.9
 Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
This breadth allows customers to consolidate their ion channel research needs with a single provider, streamlining project management and communication, and enabling them to benefit from:
- Industry-leading expertise: A team of highly skilled scientists with extensive knowledge of ion channel pharmacology
- State-of-the-art technology: Access to automated patch clamp systems for high-throughput screening
- Gold standard electrophysiology: Experts in all in vitro manual patch clamp configurations
- Extensive collection of ion channel cell lines: Access to broad range of ion channel targets for selectivity assessment, including CiPA panel
- Customised research solutions: Tailored services designed to meet specific project needs in both in vitro and translational assays
- Fast and reliable data reporting: Accelerating the pace of drug discovery with rapid turnaround times
- Translational assays: Validation of lead compounds in sensory neurons
- Comprehensive scientific support: Providing detailed data interpretation and expert guidance
Take the next step: Accelerate your drug discovery pipeline with Metrion
Don’t let NaV1.9 screening challenges stall your pipeline. Partner with Metrion to access reliable, high-throughput solutions designed for success - contact us today:
Reference
- https://market.us/report/chronic-pain-treatment-market/
Access high-value, underexploited pain targets
The global chronic pain treatment market was valued at $88.2 billion in 2023 and is projected to reach $173.4 billion by 2033, growing at a 7.0% CAGR. Neuropathic pain, a major segment of this market, represents around 31.6% of the total.1
NaV1.9 is a key ion channel target for pain drug discovery, particularly for development of novel non-opioid treatments for chronic pain. However, the path to progressing therapies targeting NaV1.9 has been blocked by lack of robust and reliable NaV1.9 high-throughput screening assays.
Metrion has overcome these obstacles with a NaV1.9 screening cascade, built upon a robust and stable hNaV1.9 cell line, using automated patch-clamp technology and translational assays. This solution enables:
- Clearer vision of growth for pain drug discovery pipeline and develop more effective treatments for chronic pain in this under explored and clinically relevant area
- High-quality reproducible data allowing rapid hit identification, hit expansion and SAR.
- Consistent and reproducible results.

Unique approach to NaV1.9 drug discovery for faster, smarter, cost-effective screening
Our NaV1.9 suite of services supported by highly experienced electrophysiologists, lead by a Leadership team with over 100 years’ experience in drug discovery research, sets the Metrion service apart from others. The business case is clear for organisations looking to rapidly meet project milestones:
- Shorter timelines: High-quality screening assays accelerate project milestones.
- Lower costs: Reliable screening data to robustly support medicinal chemistry.
- Cost-effective outsourcing: Rapid, expert data interpretation ensures confident advancement of promising compounds.
- High confidence in NaV1.9 screening cascade:
- Identify high quality hits
- Accelerate hit-to-lead discovery
- Investigate mechanism of action via translational assays
For business leaders, this translates into:

Figure 1. Example of Metrion's NaV1.9 screening cascade.
Reliable, high-throughput NaV1.9 screening for faster, consistent and accurate results
Our validated, high-throughput NaV1.9 screening solution delivers consistent and accurate results. Key advantages:
- High-throughput NaV1.9 screening for rapid compound testing: The CHO-hNaV1.9 cell line, developed in-house by Metrion, enables automated patch-clamp screening using the Qube 384 platform. This system allows rapid testing of large compound libraries and hit-to-lead support.
- Reliable NaV1.9 data, fewer setbacks: The stable and robust expression of Metrion’s NaV1.9 cell line ensures high reproducibility and low assay variability. We have demonstrated high sensitivity, robustness and accuracy of NaV1.9 screening data during the assay development with the testing of tool compounds and compound plates randomly spiked with a NaV1.9 inhibitor.
The business challenge: Missed opportunities
The NaV1.9 ion channel is a promising target for developing non-opioid chronic pain treatments, but challenges in establishing screening assays have slowed progress across the industry. Key issues include:
- Functional expression challenges: difficulty in expressing NaV1.9 in stable heterologous systems.
- Biophysical properties: activation and inactivation kinetics of NaV1.9 require voltage-control only offered by automated electrophysiological techniques.
Metrion's unique approach overcomes these challenges and unlocks the potential for increased ROI in pain drug discovery.
Choose Metrion as your CRO: Contract research services for preclinical drug discovery beyond NaV1.9
 Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
This breadth allows customers to consolidate their ion channel research needs with a single provider, streamlining project management and communication, and enabling them to benefit from:
- Industry-leading expertise: A team of highly skilled scientists with extensive knowledge of ion channel pharmacology
- State-of-the-art technology: Access to automated patch clamp systems for high-throughput screening
- Gold standard electrophysiology: Experts in all in vitro manual patch clamp configurations
- Extensive collection of ion channel cell lines: Access to broad range of ion channel targets for selectivity assessment, including CiPA panel
- Customised research solutions: Tailored services designed to meet specific project needs in both in vitro and translational assays
- Fast and reliable data reporting: Accelerating the pace of drug discovery with rapid turnaround times
- Translational assays: Validation of lead compounds in sensory neurons
- Comprehensive scientific support: Providing detailed data interpretation and expert guidance
Take the next step: Accelerate your drug discovery pipeline with Metrion
Don’t let NaV1.9 screening challenges stall your pipeline. Partner with Metrion to access reliable, high-throughput solutions designed for success - contact us today:
Reference
- https://market.us/report/chronic-pain-treatment-market/


 Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.

 Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.

 Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.