Using automated patch clamp technology, we evaluate the potency and selectivity of ten Nav1.7-selective arachnid peptide toxins, which have been fused to the C-terminus (Fc region) of human IgG1.
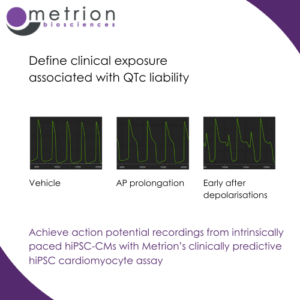 Early cardiac derisking: A clinically translatable hiPSC cardiomyocyte assay
Early cardiac derisking: A clinically translatable hiPSC cardiomyocyte assayAchieve action potential recordings from intrinsically paced hiPSC-CMs with Metrion’s clinically predictive hiPSC cardiomyocyte assay.
Read more about our hiPSC cardiomyocyte assay.
Using automated patch clamp technology, we evaluate the potency and selectivity of ten Nav1.7-selective arachnid peptide toxins, which have been fused to the C-terminus (Fc region) of human IgG1.
Understanding cardiac safety early is critical in drug development. In their latest poster, Jazz Pharmaceuticals, explain how they utilised Metrion’s clinically translatable cardiotoxicity assay to do exactly that.