Using automated patch clamp technology, we evaluate the potency and selectivity of ten Nav1.7-selective arachnid peptide toxins, which have been fused to the C-terminus (Fc region) of human IgG1.
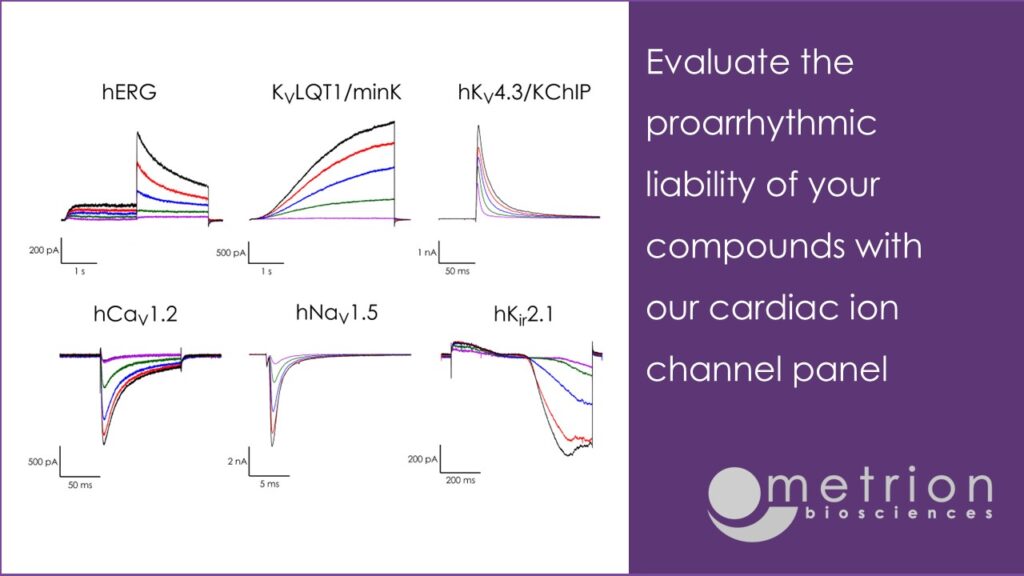
Improve efficiency, reduce late-stage failures, and align with regulatory standards by assessing the proarrhythmic liability of your compounds early. The potency data derived from high-fidelity platforms such as automated patch-clamp and the gold standard manual patch-clamp technique, is suitable for use in in silico action potential models. Our full cardiac ion channel panel includes: hERG (including a robust, dynamic hERG assay), KVLQT1/mink, hKV4.3/KChIP, hCaV1.2, hNaV1.5 (peak and late), hKIR2.1. Screening services against hHCN4 and hKV1.5, which play important roles in controlling human heart rate and atrial repolarisation, respectively, are also provided.
Learn more about our cardiac ion channel panel.
Using automated patch clamp technology, we evaluate the potency and selectivity of ten Nav1.7-selective arachnid peptide toxins, which have been fused to the C-terminus (Fc region) of human IgG1.
Understanding cardiac safety early is critical in drug development. In their latest poster, Jazz Pharmaceuticals, explain how they utilised Metrion’s clinically translatable cardiotoxicity assay to do exactly that.