Using automated patch clamp technology, we evaluate the potency and selectivity of ten Nav1.7-selective arachnid peptide toxins, which have been fused to the C-terminus (Fc region) of human IgG1.
Validating compounds in translational assays is vital to progressing your ion channel drug discovery programme. We offer a range of translational, phenotypic neuronal assays and platforms employing native rodent and human iPSCs from the central nervous system (CNS). These assays are useful for determining the efficacy, potency and mechanism-of-action of customer compounds designed to treat CNS diseases such as epilepsy, anxiety, depression and neurodegeneration , as well as determining potential CNS toxicity issues for ligands expected to penetrate into the brain.
With a focus on electrophysiological readouts, we use the gold standard manual patch (voltage and current clamp) and multi-electrode array (MEA) platforms to record changes in single cell and neuronal network activity, and determine the effects of media, cell biology modulators, signalling pathways and test compounds.
We can design these assays specifically for your translational neuroscience and drug discovery needs.
Multi-electrode arrays (MEA) are a powerful tool to investigate the neuronal firing fingerprint of your cells of choice over days, weeks or even months. Neurons are cultured over a large set of electrodes and over time, a network is established with distinct characteristics. Extracellular field potentials are recorded, providing a non-invasive way of monitoring neuronal activity. Cells from different origins can be compared and / or effects of compounds on excitability, connectivity and synchrony investigated.
Applications of this technology include examining potential neurotoxicity of compounds, thereby helping to define safety margins. Alternatively, MEA recordings can be used to verify the desired actions of compounds specifically developed to be active in the central nervous system (CNS).
We use two different MEA systems:
Axion Maestro: a high-throughput device with a total of 768 electrodes that can be utilised in 12, 48 or 96 well formats.
MED64 platform from Alpha MED: a lower throughput but highly flexible instrument with 64 electrodes that is better suited to certain applications.
To further investigate the effects of a compound high fidelity single cell manual patch-clamp recordings can be employed. Current-clamp is an information rich technique which records passive membrane properties and firing behaviour from individual neurons in response to current input and / or compound application. This data can be used to verify work from other sources and then drill down to determine the mechanism of action (MOA) of the compound in question. For instance, a state-dependent sodium channel blocker is likely to limit multiple action potential firing (as shown in Fig. 1). Further in-depth analysis of action potential shape can give further MOA information.
At Metrion, we have multiple manual patch-clamp rigs available with highly trained and experienced operatives. We aim to be able to provide an integrated service where compounds can be investigated using multiple techniques (such as linking MEA work with current-clamp), and to also provide safety assays to allow assessment of the potential therapeutic window. Subsequent to current-clamp work, Metrion can also follow-up with efficacy studies against specific ion channel targets using voltage clamp recordings from native neurons or heterologous cell lines.
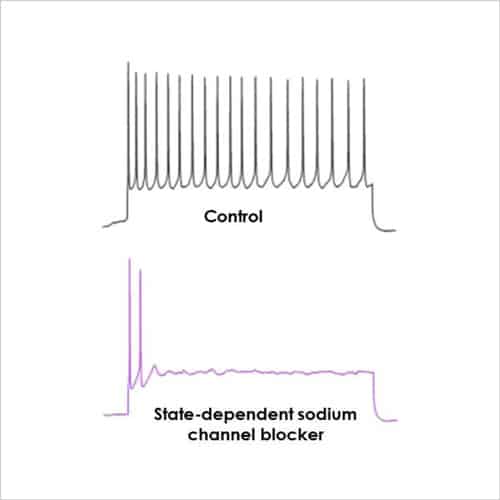
Figure 1. Current-clamp recording
As nervous system side-effects continue to be one of the major causes of drug attrition, it is critical that new drug candidates are tested in predictive neurotoxicity assays.
Neurotoxicity screening can be carried out efficiently and reliably in plate-based assays such as the multi-electrode array (MEA) using peripheral and CNS neurons from native tissue or human iPSC-derived cells. Spiking activity is closely monitored over extended periods using biophysical techniques able to detect acute and chronic effects of test compounds under physiological conditions.
Multi-electrode array platforms enables simultaneous recording of the physiological activity from multiple neuronal cells and complex networks. Extracellular field potentials are recorded in a non-invasive manner to characterise neuronal firing before and after compound addition, detecting changes in single neuron activity as well as higher level network synchronisation. The electrophysiological behaviour of populations of disease modified neurons or cells from different sources can also be compared with relative ease.
We have validated an industry-standard rat cortical CNS neuron excitability assay on a MEA platform (here) that can reliably detect inhibitory and excitatory effects of a wide range of agricultural and medical agents. Alongside the FDA’s NeuTox initiative we are also developing translational CNS seizure liability assays using human iPSC-derived CNS neurons.
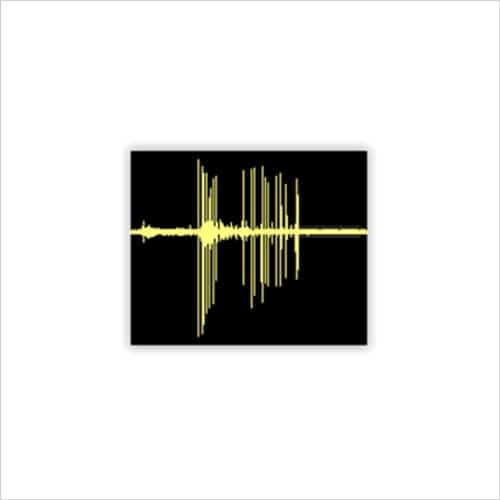
Figure 2a. Cortical neuron firing on MEA
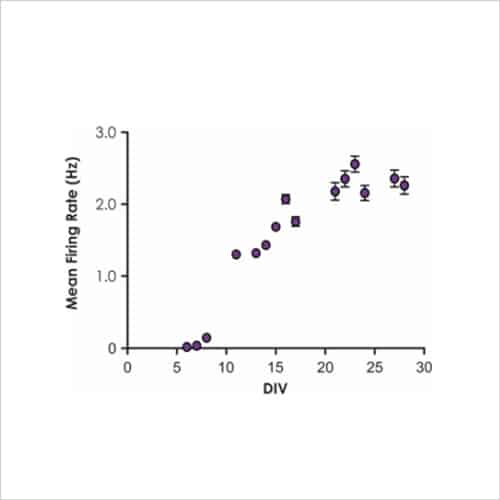
Figure 2b. Current-clamp recording
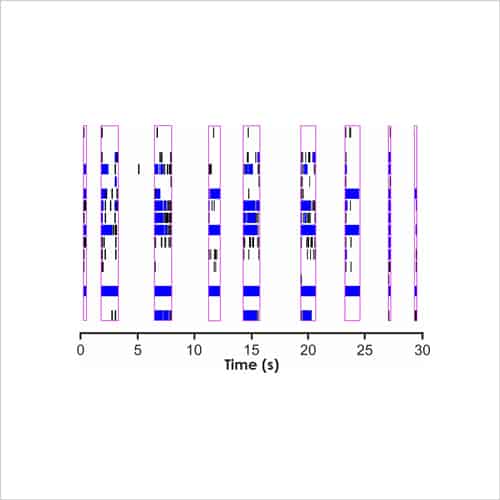
Figure 3a. Current-clamp recording
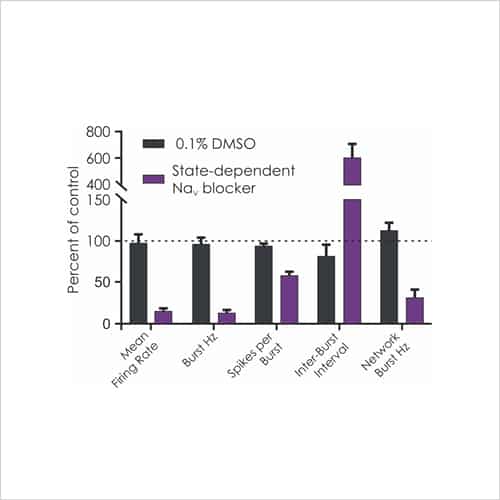
Figure 3b. Effects of Nav inhibitor on firing parameters
Rat cortical neurons cultured on MEA plates increase their activity to become more co-ordinated and stable over time to match that of postnatal animals (Fig. 2b), after which time test compounds can be assessed for complex effects on cell firing and network bursting (Fig. 3b).
Using automated patch clamp technology, we evaluate the potency and selectivity of ten Nav1.7-selective arachnid peptide toxins, which have been fused to the C-terminus (Fc region) of human IgG1.
Development of a robust hNaV1.9 high-throughput screening assay on the Sophion Qube384 platform. This is complemented by a suite of ion channel selectivity assays and sensory neuron recordings to create a versatile screening cascade to support NaV1.9 drug discovery programmes.