Metrion has extensive ion channel drug discovery experience of indications such as pain, neurodegeneration, psychiatry and epilepsy.
As our specialist team of neuroscientists are experts in their field we can advance your project to the next milestone, as well as help your biology team generate cell lines for your targets.
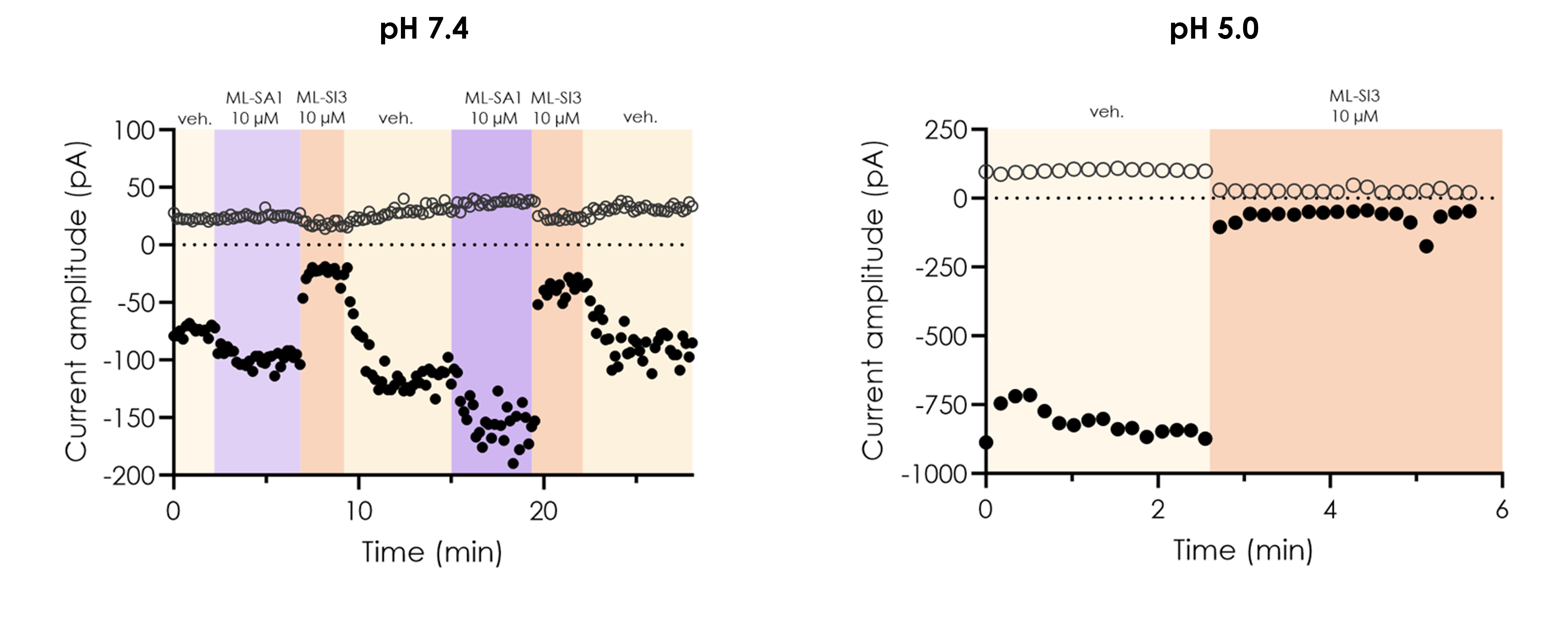
We have extensive experience in electrophysiology and ion channel screening assays, and translational assays, delivering high-quality recordings from relevant tissues expressing your target of interest.
We offer high-throughput automated patch clamp services, fluorescence, MEA and manual patch (intracellular/organelle recordings).