Metrion Biosciences is a leading provider of high-quality
GLP (Good Laboratory Practice) hERG testing services, designed to meet the stringent needs of pharmaceutical and biotech companies involved in drug discovery. With a strong focus on delivering cost-effective solutions, Metrion ensures customers receive accurate and reliable data without compromising on quality, all within a remarkably quick turnaround time.
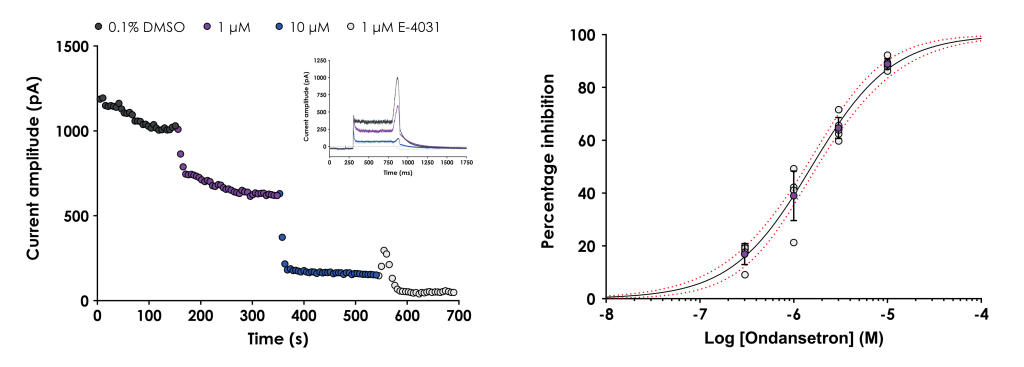
The hERG (human Ether-à-go-go-Related Gene) assay is a critical component in the early stages of drug discovery, and GLP hERG testing is vitally important for the Investigational New Drug (IND) process.