Validating compounds in translational assays is vital to progressing your ion channel drug discovery programme. We offer a range of translational, phenotypic neuronal assays and platforms employing native rodent and human iPSCs from the central nervous system (CNS). These assays are useful for determining the efficacy, potency and mechanism-of-action of customer compounds designed to treat CNS diseases such as epilepsy, anxiety, depression and neurodegeneration , as well as determining potential CNS toxicity issues for ligands expected to penetrate into the brain.
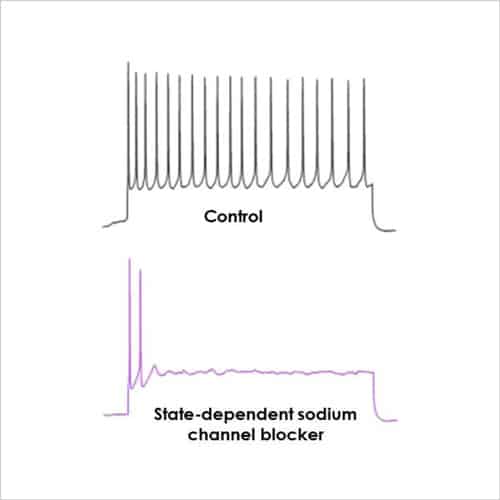
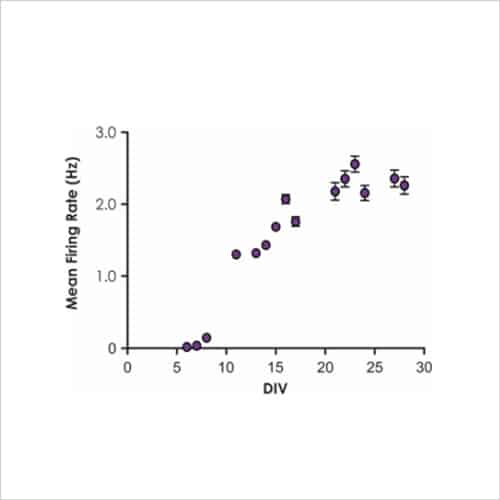
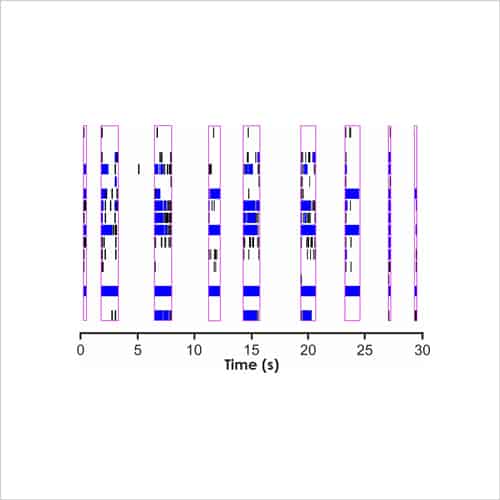
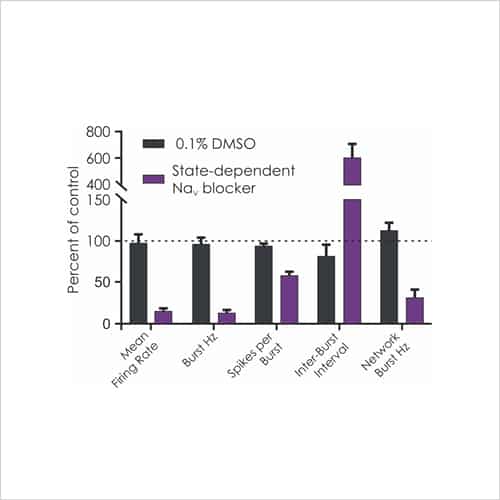
With a focus on electrophysiological readouts, we use the gold standard manual patch (voltage and current clamp) and multi-electrode array (MEA) platforms to record changes in single cell and neuronal network activity, and determine the effects of media, cell biology modulators, signalling pathways and test compounds.
We can design these assays specifically for your translational neuroscience and drug discovery needs.