Understanding cardiac safety early is critical in drug development. In their latest poster, Jazz Pharmaceuticals, explain how they utilised Metrion’s clinically translatable cardiotoxicity assay to do exactly that.
 Reduce the chance of late-stage failures due to cardiac toxicity
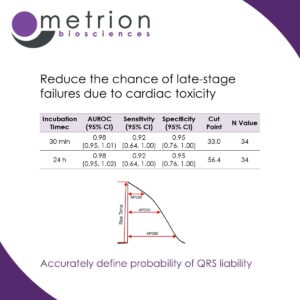
Reduce the chance of late-stage failures due to cardiac toxicityDefining the exposure associated with QRS prolongation probability is essential for assessing cardiovascular risk in drug discovery, as it helps identify compounds that may cause ventricular conduction delays and increase the likelihood of life-threatening arrhythmias.
Prolongation of the QRS interval indicates impaired ventricular depolarisation, often due to sodium channel (NaV1.5) blockade, which can lead to conditions like bundle branch block or re-entrant arrhythmias. Regulatory agencies consider significant QRS widening a key safety concern as it is associated with an elevated risk of sudden cardiac events.
By accurately defining the drug exposure levels that affect QRS duration, researchers can establish safety margins, prioritise lower-risk compounds, and reduce the chance of late-stage failures due to cardiac toxicity. This proactive approach improves drug development efficiency and enhances patient safety.
Read more about our hiPSC cardiomyocyte assay.
Understanding cardiac safety early is critical in drug development. In their latest poster, Jazz Pharmaceuticals, explain how they utilised Metrion’s clinically translatable cardiotoxicity assay to do exactly that.
Aligos Therapeutics and Metrion explore key approaches to cardiovascular safety screening in drug discovery.