Understanding cardiac safety early is critical in drug development. In their latest poster, Jazz Pharmaceuticals, explain how they utilised Metrion’s clinically translatable cardiotoxicity assay to do exactly that.
Using the conventional whole-cell patch-clamp technique, the gold standard for hERG testing, Metrion’s GLP hERG testing services are designed to help pharmaceutical companies and biotech organisations ensure the safety and efficacy of their compounds during the drug development process.
This specialist Good Laboratory Practice (GLP) testing service assesses the potential of new chemical entities (NCEs) to inhibit the hERG (human ether-à-go-go related gene) potassium channel. The hERG channel is a critical component of cardiac function, and its inhibition can lead to potentially fatal arrhythmias. Therefore, our testing services are not only crucial for the safety of a drug but also for its regulatory approval. GLP compliant hERG testing is mandatory under the ICH S7B guidelines, which states that in vitro IKr and in vivo QT assays described in Sections 2.3.1 and 2.3.2 when performed for regulatory submission should be conducted in compliance with good laboratory practice (GLP).
Testing is carried out in our GLP accredited laboratory, in compliance with the guidelines set out by the International Council for Harmonisation (ICH) S7B guidelines and is performed in accordance with Good Laboratory Practice (GLP) principles, ensuring that our data is reliable, reproducible, and meets the necessary regulatory standards.
Metrion provides GLP-compliant hERG testing services using the conventional whole-cell patch-clamp technique, which is the standard method for assessing hERG channel inhibition. The patch-clamp technique allows for precise measurement of ion currents across individual cell membranes, making it ideal for studying the effects of drugs on ion channel function.
Our hERG assay services are carried out in compliance with the FDA’s best practice guidelines, as well as the UK GLP regulations (SI 1999 No. 3106; amendments, SI 2004 No. 994) and the OECD No. 1 principles on Good Laboratory Practice. Our laboratories operate at physiological temperatures (36 ± 2 °C), and we follow the FDA’s recommended voltage protocol and experimental solutions to ensure consistency and accuracy in the results.
GLP hERG testing is a critical component in the preclinical safety evaluation of new chemical entities. The human ether-à-go-go-related gene (hERG) encodes a key potassium ion channel that plays an essential role in the repolarisation phase of the cardiac action potential. Any alterations in the function of these channels can lead to severe cardiac arrhythmias and potentially fatal outcomes. As such, a robust hERG assay is indispensable in predicting cardiotoxicity risk.
This case study details GLP hERG Assay Validation Following ICH E14/S7B 2022 Q&A Best Practice Guidelines, ensuring that our methods deliver high-quality, reproducible and transparent data.
As part of our GLP-compliant services, we offer dose formulation analysis (DFA), which is an obligatory requirement for GLP studies submitted in IND filings. DFA is performed in collaboration with our preferred GLP-accredited partner, who is responsible for verifying the concentration of dosing formulations used in our hERG assays. This ensures that the compounds being tested are accurately dosed and that the results of our experiments are scientifically valid.
One of the key contributions of Metrion to the field of hERG testing is our involvement in research that helps support integrated QTc (corrected QT interval) risk assessment. In a recent study, we co-authored a paper examining the inter-laboratory variability in hERG testing and assessing the hERG safety margin for three positive control agents used in cardiac safety studies. This work has contributed to a deeper understanding of the variability in hERG screening data and has helped establish more reliable safety margins for evaluating new drug candidates.
The study, titled ‘Supporting an Integrated QTc Risk Assessment Using the hERG Margin Distributions for Three Positive Control Agents Derived from Multiple Laboratories and on Multiple Occasions’, was published in the Journal of Pharmacological and Toxicological Methods (Volume 128, 2024). This paper discusses the variability in hERG data and how it can be used to assess the potential risk of QT prolongation in drugs under development. By providing insight into the consistency of hERG testing data across laboratories and experimental conditions, this research helps enhance the reliability of safety assessments for new pharmaceuticals.
Platform
Manual patch-clamp
GLP hERG test system
CHO
Temperature
36 ± 2°C
Number of test concentrations
4
Positive control
Ondansetron
Required for IND submission?
Yes
Dose-formulation Analysis (DFA)
Yes, using a GLP compliant partner
GLP-compliant hERG testing is a requirement under the ICH S7B guidelines, which mandate that certain safety pharmacology studies, including those assessing a drug’s potential interaction with the hERG potassium channel, must adhere to Good Laboratory Practice (GLP) principles.
According to these guidelines:
hERG testing is a critical component of preclinical safety pharmacology studies required for Investigational New Drug (IND) applications. It ensures that:
Our hERG testing services have been:
These audits and accreditations highlight the quality and reliability of our services, reaffirming that we operate in full compliance with the highest regulatory standards.
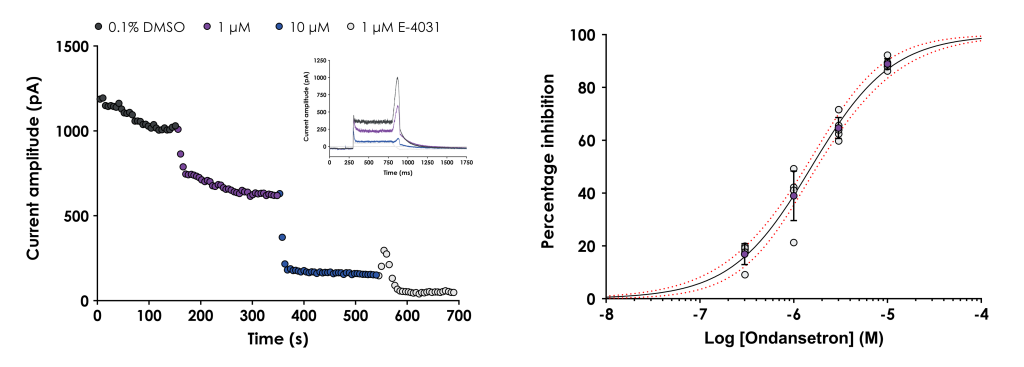
Figure 1. A. Graph showing the peak hERG tail current plotted against time for a representative cell treated with 1 and 10 μM ondansetron followed by 1 μM E-4031. The inset figure shows representative current traces in 0.1% DMSO, ondansetron and E-4031. B. Graph showing the mean ± SD inhibition data (purple symbols), as well as individual inhibition values (grey symbols), plotted against concentration. The data were fitted with a Hill equation to yield the half-maximal inhibition concentration (IC50) and the 95% confidence bands (red dashed lines).
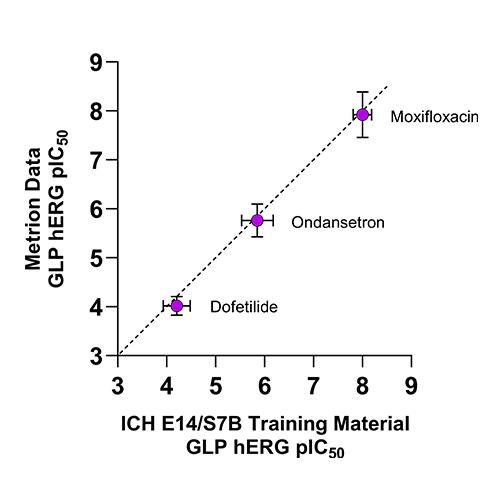
Figure 2. Comparison of GLP hERG IC50 values from Metrion versus published ICH E14/S7B training material values.
The hERG potassium channel plays a crucial role in regulating the electrical activity of the heart. It is responsible for conducting potassium ions during the repolarisation phase of the cardiac action potential.
Inhibition of this channel can lead to:
Given these risks, screening potential drug candidates for hERG channel interactions is vital to ensuring the safety of new therapeutics.
The necessity of hERG screening is underscored by past drug withdrawals and restrictions due to their ability to block the channel. Several drugs, initially deemed safe and effective, were later found to:
Early implementation of hERG screening in drug discovery has been a key factor in reducing the number of proarrhythmic drugs reaching the market, ultimately improving patient safety and lowering the incidence of drug-induced cardiac events.
Conducting GLP-compliant hERG testing early in drug development is crucial as it:
Having reliable and compliant hERG screening data is essential for advancing drug candidates through regulatory approval, ensuring both safety and success in drug development.
hERG testing is a critical component of the safety pharmacology profile of any new drug. The ICH S7A and S7B guidelines provide regulatory guidance on performing safety pharmacology studies for human pharmaceuticals, with specific emphasis on the importance of assessing a compound’s interaction with the hERG channel. IND applications for small molecule drugs include pharmacological assessments of hERG inhibition, as part of the required preclinical safety testing.
Drugs that block the hERG channel can cause QT prolongation, which may lead to arrhythmias such as Torsades de Pointes. This is why the evaluation of hERG inhibition is mandatory in drug development. By using the latest patch-clamp technology, we can accurately measure the extent to which a drug inhibits the hERG channel, allowing pharmaceutical companies to make informed decisions regarding the cardiac safety of their compounds.
Metrion offers a comprehensive, GLP-compliant hERG testing service that is critical for assessing the safety of new drug candidates. Our high-quality, cost-effective, and rapid-turnaround testing is performed using the conventional whole-cell patch-clamp technique, in accordance with FDA and UK GLP guidelines. By providing reliable data on a drug’s potential to cause cardiac arrhythmias, our hERG testing services help pharmaceutical companies navigate the regulatory approval process and ensure that their compounds are safe for clinical use.
We are committed to advancing drug safety and supporting pharmaceutical development by providing reliable, high-quality hERG testing that adheres to the most stringent regulatory standards. Our expertise and experience in the field make us a trusted partner for companies looking to mitigate the risk of proarrhythmic liabilities in their drug candidates.
Understanding cardiac safety early is critical in drug development. In their latest poster, Jazz Pharmaceuticals, explain how they utilised Metrion’s clinically translatable cardiotoxicity assay to do exactly that.
Aligos Therapeutics and Metrion explore key approaches to cardiovascular safety screening in drug discovery.