An important role for hiPSC-cardiomyocytes in assessing cardiac risk
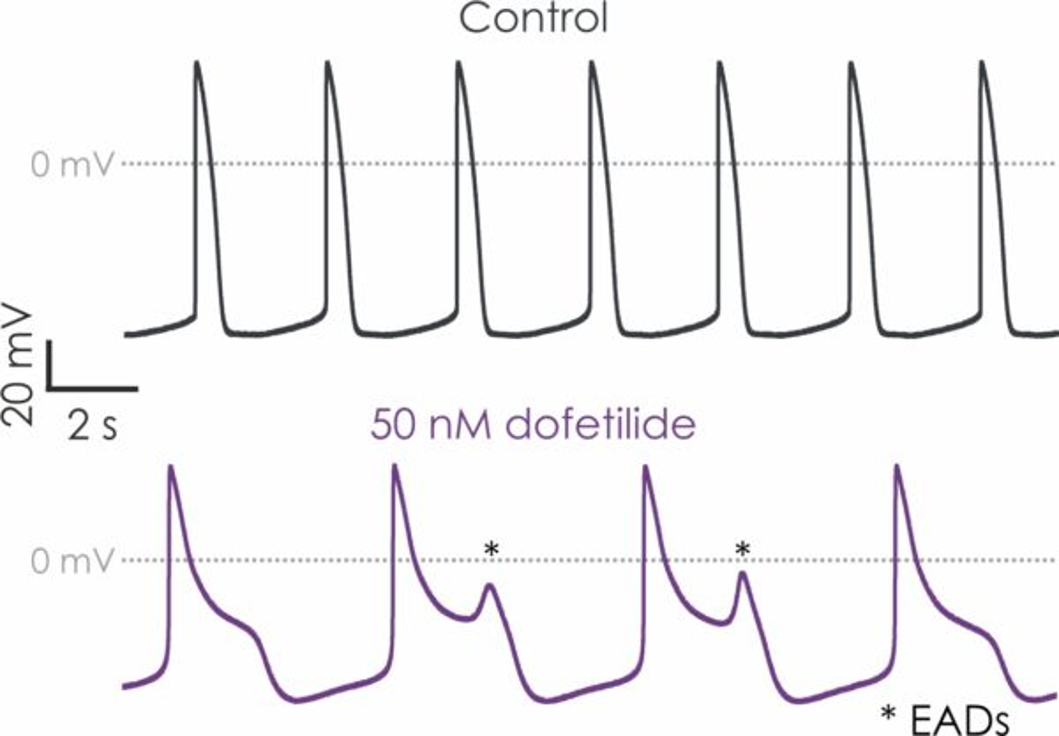
Proarrhythmia is a serious concern in drug development, as many pharmaceuticals - particularly those targeting the heart or central nervous system - can alter the normal rhythm of the heart. These drugs may affect the electrical properties of cardiomyocytes, leading to prolonged or shortened action potentials, delayed repolarisation, or other disturbances that can result in arrhythmias.
Identifying drugs that pose a proarrhythmic risk early in the development process is critical to reducing harm and improving patient safety. Traditional in vivo models are often inadequate in predicting human-specific cardiac responses. They fail to capture the complexity of human cardiac electrophysiology and often lead to unreliable or inconclusive results. This is where hiPSC-derived cardiomyocytes are can play a key role for testing proarrhythmic potential.
hiPSC-derived cardiomyocytes are stem cell-derived heart cells that mimic human cardiac function more closely than animal-derived cells. These cells enable researchers to examine drug effects on human-specific cardiac ion channels and electrophysiological behaviour, giving them more accurate and reliable data than conventional models. By evaluating drug effects on these human-relevant cells, researchers can better predict the likelihood of arrhythmias and reduce the risk of adverse cardiovascular events.