Figure 1. Hippocampal spontaneous post-synaptic currents (sPSCs) and current-clamp recordings of action potential firing. A. Representative bright field image of a rodent hippocampus, with the subfields cornu ammonis 1 (CA1) and 3 (CA3), and dentate gyrus (DG) indicated. A representative neuron from CA1 (indicated within the box) is shown with a glass recording electrode attached during an experiment (right panel). B. Representative recordings of spontaneous post-synaptic currents at -70 mV holding potential. The inset illustrates a zoomed-in area of the recording. C. Action potential responses to 1-second pulses of injected current as indicated in the current-clamp protocol (upper panel).
Figure 2. Pharmacological and electrophysiological characterisation of hippocampal evoked post-synaptic currents.
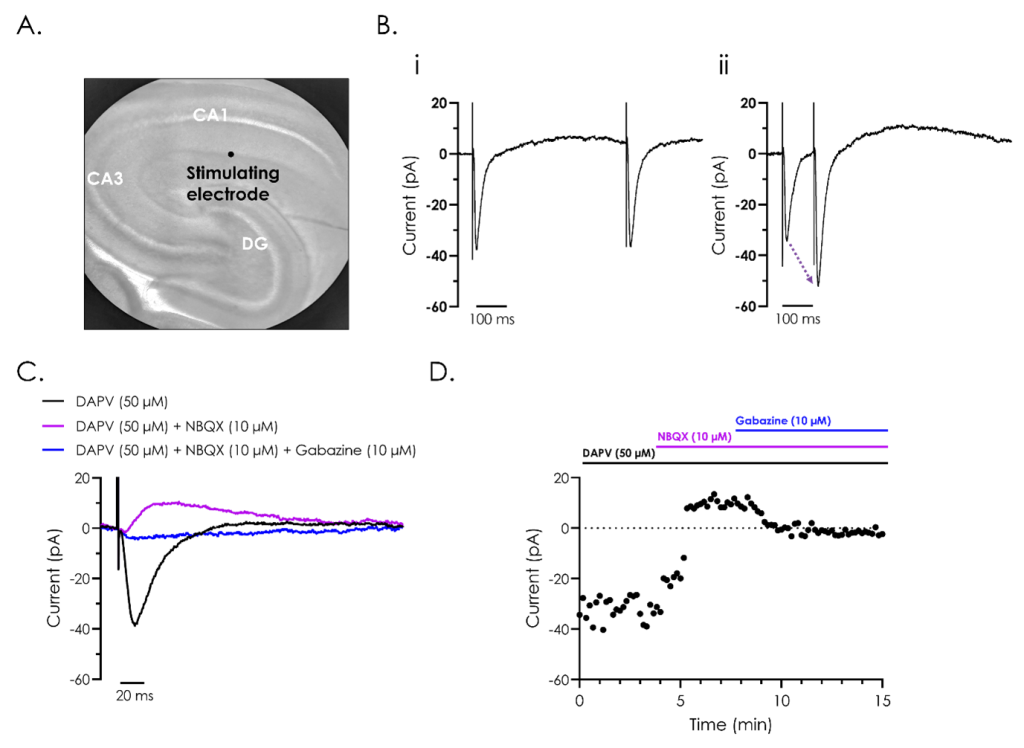
A. Representative bright field image of a rodent hippocampus (left panel), with the subfields cornu ammonis 1 (CA1) and 3 (CA3), and dentate gyrus (DG) indicated. A stimulating electrode was attached close to CA1, as illustrated.
B. A representative recording of evoked post-synaptic currents from hippocampal neurons from CA1, utilising ‘paired-pulse’ stimulation to investigate synaptic plasticity. The effect of reducing the inter-stimulus interval from 500 (i) to 100 (ii) ms illustrates synaptic facilitation, whereby the amplitude of the second evoked current is enhanced as illustrated by the dashed arrow in panel ii.
C. Representative recordings of evoked post-synaptic currents illustrating the different components which make up evoked post-synaptic currents. Recordings were performed after exposure to the NMDA receptor inhibitor D (-)-2-amino-5-phosphonvalerate (DAPV), both alone and in combination with the AMPA receptor inhibitor 2,3-dioxo-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX), and GABA antagonist, gabazine.
D. A representative current-time plot showing the peak inward and outward currents recorded from the different components of the evoked post-synaptic currents during a 15-minute recording.