As the FDA ushers in a new era of drug development by loosening its reliance on animal models for investigational new drug (IND) applications, the life sciences industry stands at a critical juncture. The FDA's April 10 announcement marks a transformative shift in preclinical testing, particularly for monoclonal antibodies and select drug candidates. By embracing New Approach Methodologies (NAMs) - which include computational models, human cell lines, and organoids - the FDA is aligning regulatory science with ethical imperatives and scientific innovation.
 One technology that stands to play a central role in this transition is Metrion’s cardiomyocyte assay, a cutting-edge tool that uses human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in conjunction with high-frequency optical recording to evaluate cardiac safety. In light of the FDA’s changing stance, this assay emerges as a powerful, validated NAM capable of addressing the very safety concerns traditionally assessed in animal studies.
One technology that stands to play a central role in this transition is Metrion’s cardiomyocyte assay, a cutting-edge tool that uses human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in conjunction with high-frequency optical recording to evaluate cardiac safety. In light of the FDA’s changing stance, this assay emerges as a powerful, validated NAM capable of addressing the very safety concerns traditionally assessed in animal studies.
Historically, the FDA required animal testing as a cornerstone of the IND process. This approach, grounded in the Food, Drug, and Cosmetic Act of 1938, was intended to protect human subjects from unforeseen toxicities. However, the advent of advanced human-relevant models has made this paradigm increasingly outdated.
The FDA Modernization Act 2.0, passed in 2022, opened the door for non-animal testing methods, and the pending 3.0 version aims to ensure timely implementation. While full replacement of animal models is not yet mandated, the FDA now permits sponsors to submit validated NAM data in lieu of animal results on a case-by-case basis, signalling a phased, pragmatic approach.
FDA Commissioner Marty Makary, M.D., described the shift as a "win-win for public health and ethics," highlighting the promise of AI-based modelling and organ-on-chip technologies. However, concerns remain. Industry stakeholders like the National Association for Biomedical Research (NABR) argue that no single NAM can yet replicate the complexity of whole-organism biology. Surveys also show hesitancy in the R&D community, with 60% of professionals wary of regulatory ambiguity around non-animal methods.
This is where Metrion’s cardiomyocyte assay has the potential to offer both clarity and capability for preclinical drug discovery.
The cadiomyocyte assay leverages hiPSC-CMs to replicate human cardiac electrophysiology and less clinically translational ex vivo animal model. Using a high-throughput 96-well format and a voltage-sensitive fluorescent dye, it enables simultaneous action potential recordings at a high frequency (10kHz) using the Lumencor VOLTA plate reader. The assay can assess multiple cardiac safety endpoints, including:
These are the very biomarkers used in evaluating the cardiac safety of drugs, traditionally assessed in vivo through animal telemetry or ex vivo in isolated heart systems. Unlike animal models, however, our cardiomycyte assay offers human relevance, reproducibility and scalability.
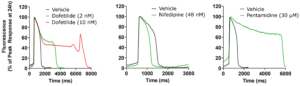
Figure 1. Representative traces showing the impact on hiPSC-derived action potential morphology of selective ion channel or hERG trafficking block. Metrion’s hiPSC-derived cardiomyocyte assay is a powerful tool for early cardiac derisking, contributing to safer and more efficient drug development processes.
Crucially, this model can predict a compound’s potential to prolong the QTc interval, a known risk factor for arrhythmias such as Torsades de Pointes. Additionally, it can predict the free clinical exposure associated with a 10 ms QTc increase - an FDA-recognised threshold for concern under ICH E14/S7B guidelines.
The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, supported by the FDA, aimed to replace the traditional hERG assay and animal studies with a more integrated approach to cardiac safety. The cardiomyocyte assay aligns perfectly with CiPA’s framework by using a human cellular model that can capture complex electrophysiological effects, not just ion channel block.
Where ion channel testing might miss liabilities caused by non-ion channel mechanisms, the cardiomyocyte assay can reveal them. This capacity is particularly important for biologics like monoclonal antibodies, which may not exhibit classical small-molecule toxicity patterns but can still affect cardiac electrophysiology via indirect pathways.
While the FDA's gradual move away from animal testing is encouraging, the pathway to full adoption of NAMs depends heavily on validation and regulatory confidence. The cardiomyocyte assay addresses this by enhancing preclinical drug discovery with:
These features not only align with the FDA’s future vision but provide sponsors with confidence that their preclinical safety data can withstand regulatory scrutiny.
Moreover, our cardiomyocyte assays utility extends beyond regulatory submission. It can be used in early-stage discovery to de-risk compounds before resource-intensive development, saving both time and cost.
Despite regulatory momentum, many pharmaceutical companies remain hesitant to abandon animal models. Concerns around regulatory acceptance and the complexity of biological systems persist, especially among established players represented by NABR and PhRMA.
Our cardiomyocyte assay offers a practical middle ground: a validated, translational model that provides rich safety data while adhering to the principles of the "three Rs" of animal use:
Animal advocacy groups such as PETA have applauded the FDA's direction and are likely to support wider adoption of platforms like Volta. Likewise, smaller biotech companies - often more nimble in adopting new technologies - may see this as an opportunity to accelerate IND timelines without the ethical and financial burden of animal testing.
The FDA plans to hold a public workshop later this year to further explore the implementation of NAMs. A pilot program is already underway, with select drugmakers testing antibodies using non-animal methods in close consultation with the agency.
For innovators in the NAM space, this represents a pivotal opportunity. The cardiomyocyte assay, with its ability to generate high-fidelity, human-relevant cardiac safety data, is poised to become a benchmark tool in this new regulatory ecosystem.
As regulatory bodies around the world follow the FDA’s lead, and as NAMs gain broader acceptance, platforms like the Volta won’t just supplement animal studies - they will replace them. And in doing so, they will help build a safer, faster, and more humane drug development pipeline.
The FDA’s endorsement of non-animal methodologies marks a monumental shift in preclinical drug discovery. With a strong foundation in human biology, high-throughput scalability, and predictive accuracy, the cardiomyocyte assay stands at the forefront of this transition. As the industry navigates this paradigm shift, tools like Volta will be indispensable - not only for regulatory compliance but for advancing science that is ethical, efficient, and firmly rooted in the future.
Now is the time to embrace innovation and align your drug development strategy with the evolving regulatory landscape. Whether you're looking to enhance preclinical safety screening, reduce reliance on animal testing, or accelerate IND submissions, the Metrion cardiomyocyte assay delivers actionable, high-fidelity data that supports confident decision-making.
Contact us to integrate this into your pipeline and help you lead the way in a post-animal testing era:
As the FDA ushers in a new era of drug development by loosening its reliance on animal models for investigational new drug (IND) applications, the life sciences industry stands at a critical juncture. The FDA's April 10 announcement marks a transformative shift in preclinical testing, particularly for monoclonal antibodies and select drug candidates. By embracing New Approach Methodologies (NAMs) - which include computational models, human cell lines, and organoids - the FDA is aligning regulatory science with ethical imperatives and scientific innovation.
 One technology that stands to play a central role in this transition is Metrion’s cardiomyocyte assay, a cutting-edge tool that uses human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in conjunction with high-frequency optical recording to evaluate cardiac safety. In light of the FDA’s changing stance, this assay emerges as a powerful, validated NAM capable of addressing the very safety concerns traditionally assessed in animal studies.
One technology that stands to play a central role in this transition is Metrion’s cardiomyocyte assay, a cutting-edge tool that uses human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in conjunction with high-frequency optical recording to evaluate cardiac safety. In light of the FDA’s changing stance, this assay emerges as a powerful, validated NAM capable of addressing the very safety concerns traditionally assessed in animal studies.
Historically, the FDA required animal testing as a cornerstone of the IND process. This approach, grounded in the Food, Drug, and Cosmetic Act of 1938, was intended to protect human subjects from unforeseen toxicities. However, the advent of advanced human-relevant models has made this paradigm increasingly outdated.
The FDA Modernization Act 2.0, passed in 2022, opened the door for non-animal testing methods, and the pending 3.0 version aims to ensure timely implementation. While full replacement of animal models is not yet mandated, the FDA now permits sponsors to submit validated NAM data in lieu of animal results on a case-by-case basis, signalling a phased, pragmatic approach.
FDA Commissioner Marty Makary, M.D., described the shift as a "win-win for public health and ethics," highlighting the promise of AI-based modelling and organ-on-chip technologies. However, concerns remain. Industry stakeholders like the National Association for Biomedical Research (NABR) argue that no single NAM can yet replicate the complexity of whole-organism biology. Surveys also show hesitancy in the R&D community, with 60% of professionals wary of regulatory ambiguity around non-animal methods.
This is where Metrion’s cardiomyocyte assay has the potential to offer both clarity and capability for preclinical drug discovery.
The cadiomyocyte assay leverages hiPSC-CMs to replicate human cardiac electrophysiology and less clinically translational ex vivo animal model. Using a high-throughput 96-well format and a voltage-sensitive fluorescent dye, it enables simultaneous action potential recordings at a high frequency (10kHz) using the Lumencor VOLTA plate reader. The assay can assess multiple cardiac safety endpoints, including:
These are the very biomarkers used in evaluating the cardiac safety of drugs, traditionally assessed in vivo through animal telemetry or ex vivo in isolated heart systems. Unlike animal models, however, our cardiomycyte assay offers human relevance, reproducibility and scalability.

Figure 1. Representative traces showing the impact on hiPSC-derived action potential morphology of selective ion channel or hERG trafficking block. Metrion’s hiPSC-derived cardiomyocyte assay is a powerful tool for early cardiac derisking, contributing to safer and more efficient drug development processes.
Crucially, this model can predict a compound’s potential to prolong the QTc interval, a known risk factor for arrhythmias such as Torsades de Pointes. Additionally, it can predict the free clinical exposure associated with a 10 ms QTc increase - an FDA-recognised threshold for concern under ICH E14/S7B guidelines.
The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, supported by the FDA, aimed to replace the traditional hERG assay and animal studies with a more integrated approach to cardiac safety. The cardiomyocyte assay aligns perfectly with CiPA’s framework by using a human cellular model that can capture complex electrophysiological effects, not just ion channel block.
Where ion channel testing might miss liabilities caused by non-ion channel mechanisms, the cardiomyocyte assay can reveal them. This capacity is particularly important for biologics like monoclonal antibodies, which may not exhibit classical small-molecule toxicity patterns but can still affect cardiac electrophysiology via indirect pathways.
While the FDA's gradual move away from animal testing is encouraging, the pathway to full adoption of NAMs depends heavily on validation and regulatory confidence. The cardiomyocyte assay addresses this by enhancing preclinical drug discovery with:
These features not only align with the FDA’s future vision but provide sponsors with confidence that their preclinical safety data can withstand regulatory scrutiny.
Moreover, our cardiomyocyte assays utility extends beyond regulatory submission. It can be used in early-stage discovery to de-risk compounds before resource-intensive development, saving both time and cost.
Despite regulatory momentum, many pharmaceutical companies remain hesitant to abandon animal models. Concerns around regulatory acceptance and the complexity of biological systems persist, especially among established players represented by NABR and PhRMA.
Our cardiomyocyte assay offers a practical middle ground: a validated, translational model that provides rich safety data while adhering to the principles of the "three Rs" of animal use:
Animal advocacy groups such as PETA have applauded the FDA's direction and are likely to support wider adoption of platforms like Volta. Likewise, smaller biotech companies - often more nimble in adopting new technologies - may see this as an opportunity to accelerate IND timelines without the ethical and financial burden of animal testing.
The FDA plans to hold a public workshop later this year to further explore the implementation of NAMs. A pilot program is already underway, with select drugmakers testing antibodies using non-animal methods in close consultation with the agency.
For innovators in the NAM space, this represents a pivotal opportunity. The cardiomyocyte assay, with its ability to generate high-fidelity, human-relevant cardiac safety data, is poised to become a benchmark tool in this new regulatory ecosystem.
As regulatory bodies around the world follow the FDA’s lead, and as NAMs gain broader acceptance, platforms like the Volta won’t just supplement animal studies - they will replace them. And in doing so, they will help build a safer, faster, and more humane drug development pipeline.
The FDA’s endorsement of non-animal methodologies marks a monumental shift in preclinical drug discovery. With a strong foundation in human biology, high-throughput scalability, and predictive accuracy, the cardiomyocyte assay stands at the forefront of this transition. As the industry navigates this paradigm shift, tools like Volta will be indispensable - not only for regulatory compliance but for advancing science that is ethical, efficient, and firmly rooted in the future.
Now is the time to embrace innovation and align your drug development strategy with the evolving regulatory landscape. Whether you're looking to enhance preclinical safety screening, reduce reliance on animal testing, or accelerate IND submissions, the Metrion cardiomyocyte assay delivers actionable, high-fidelity data that supports confident decision-making.
Contact us to integrate this into your pipeline and help you lead the way in a post-animal testing era:
As the FDA ushers in a new era of drug development by loosening its reliance on animal models for investigational new drug (IND) applications, the life sciences industry stands at a critical juncture. The FDA's April 10 announcement marks a transformative shift in preclinical testing, particularly for monoclonal antibodies and select drug candidates. By embracing New Approach Methodologies (NAMs) - which include computational models, human cell lines, and organoids - the FDA is aligning regulatory science with ethical imperatives and scientific innovation.
 One technology that stands to play a central role in this transition is Metrion’s cardiomyocyte assay, a cutting-edge tool that uses human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in conjunction with high-frequency optical recording to evaluate cardiac safety. In light of the FDA’s changing stance, this assay emerges as a powerful, validated NAM capable of addressing the very safety concerns traditionally assessed in animal studies.
One technology that stands to play a central role in this transition is Metrion’s cardiomyocyte assay, a cutting-edge tool that uses human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in conjunction with high-frequency optical recording to evaluate cardiac safety. In light of the FDA’s changing stance, this assay emerges as a powerful, validated NAM capable of addressing the very safety concerns traditionally assessed in animal studies.
Historically, the FDA required animal testing as a cornerstone of the IND process. This approach, grounded in the Food, Drug, and Cosmetic Act of 1938, was intended to protect human subjects from unforeseen toxicities. However, the advent of advanced human-relevant models has made this paradigm increasingly outdated.
The FDA Modernization Act 2.0, passed in 2022, opened the door for non-animal testing methods, and the pending 3.0 version aims to ensure timely implementation. While full replacement of animal models is not yet mandated, the FDA now permits sponsors to submit validated NAM data in lieu of animal results on a case-by-case basis, signalling a phased, pragmatic approach.
FDA Commissioner Marty Makary, M.D., described the shift as a "win-win for public health and ethics," highlighting the promise of AI-based modelling and organ-on-chip technologies. However, concerns remain. Industry stakeholders like the National Association for Biomedical Research (NABR) argue that no single NAM can yet replicate the complexity of whole-organism biology. Surveys also show hesitancy in the R&D community, with 60% of professionals wary of regulatory ambiguity around non-animal methods.
This is where Metrion’s cardiomyocyte assay has the potential to offer both clarity and capability for preclinical drug discovery.
The cadiomyocyte assay leverages hiPSC-CMs to replicate human cardiac electrophysiology and less clinically translational ex vivo animal model. Using a high-throughput 96-well format and a voltage-sensitive fluorescent dye, it enables simultaneous action potential recordings at a high frequency (10kHz) using the Lumencor VOLTA plate reader. The assay can assess multiple cardiac safety endpoints, including:
These are the very biomarkers used in evaluating the cardiac safety of drugs, traditionally assessed in vivo through animal telemetry or ex vivo in isolated heart systems. Unlike animal models, however, our cardiomycyte assay offers human relevance, reproducibility and scalability.

Figure 1. Representative traces showing the impact on hiPSC-derived action potential morphology of selective ion channel or hERG trafficking block. Metrion’s hiPSC-derived cardiomyocyte assay is a powerful tool for early cardiac derisking, contributing to safer and more efficient drug development processes.
Crucially, this model can predict a compound’s potential to prolong the QTc interval, a known risk factor for arrhythmias such as Torsades de Pointes. Additionally, it can predict the free clinical exposure associated with a 10 ms QTc increase - an FDA-recognised threshold for concern under ICH E14/S7B guidelines.
The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative, supported by the FDA, aimed to replace the traditional hERG assay and animal studies with a more integrated approach to cardiac safety. The cardiomyocyte assay aligns perfectly with CiPA’s framework by using a human cellular model that can capture complex electrophysiological effects, not just ion channel block.
Where ion channel testing might miss liabilities caused by non-ion channel mechanisms, the cardiomyocyte assay can reveal them. This capacity is particularly important for biologics like monoclonal antibodies, which may not exhibit classical small-molecule toxicity patterns but can still affect cardiac electrophysiology via indirect pathways.
While the FDA's gradual move away from animal testing is encouraging, the pathway to full adoption of NAMs depends heavily on validation and regulatory confidence. The cardiomyocyte assay addresses this by enhancing preclinical drug discovery with:
These features not only align with the FDA’s future vision but provide sponsors with confidence that their preclinical safety data can withstand regulatory scrutiny.
Moreover, our cardiomyocyte assays utility extends beyond regulatory submission. It can be used in early-stage discovery to de-risk compounds before resource-intensive development, saving both time and cost.
Despite regulatory momentum, many pharmaceutical companies remain hesitant to abandon animal models. Concerns around regulatory acceptance and the complexity of biological systems persist, especially among established players represented by NABR and PhRMA.
Our cardiomyocyte assay offers a practical middle ground: a validated, translational model that provides rich safety data while adhering to the principles of the "three Rs" of animal use:
Animal advocacy groups such as PETA have applauded the FDA's direction and are likely to support wider adoption of platforms like Volta. Likewise, smaller biotech companies - often more nimble in adopting new technologies - may see this as an opportunity to accelerate IND timelines without the ethical and financial burden of animal testing.
The FDA plans to hold a public workshop later this year to further explore the implementation of NAMs. A pilot program is already underway, with select drugmakers testing antibodies using non-animal methods in close consultation with the agency.
For innovators in the NAM space, this represents a pivotal opportunity. The cardiomyocyte assay, with its ability to generate high-fidelity, human-relevant cardiac safety data, is poised to become a benchmark tool in this new regulatory ecosystem.
As regulatory bodies around the world follow the FDA’s lead, and as NAMs gain broader acceptance, platforms like the Volta won’t just supplement animal studies - they will replace them. And in doing so, they will help build a safer, faster, and more humane drug development pipeline.
The FDA’s endorsement of non-animal methodologies marks a monumental shift in preclinical drug discovery. With a strong foundation in human biology, high-throughput scalability, and predictive accuracy, the cardiomyocyte assay stands at the forefront of this transition. As the industry navigates this paradigm shift, tools like Volta will be indispensable - not only for regulatory compliance but for advancing science that is ethical, efficient, and firmly rooted in the future.
Now is the time to embrace innovation and align your drug development strategy with the evolving regulatory landscape. Whether you're looking to enhance preclinical safety screening, reduce reliance on animal testing, or accelerate IND submissions, the Metrion cardiomyocyte assay delivers actionable, high-fidelity data that supports confident decision-making.
Contact us to integrate this into your pipeline and help you lead the way in a post-animal testing era: