Results from automated patch clamp electrophysiology and gigaseal studies
Table 1. Correlation between salt pair solubility product constants (Ksp) and gigaseal formation on Qube 384. Little correlation was found between the solubility product constants (Ksp) of Ca2+, Ba2+ and Sr2+ salts (A) and their ability to foster gigaseal formation (B). Despite the PO43- salts having very low Ksp values and SrCO3 having a similar Ksp value to CaF2 and BaSO4, these salts failed to produce gigaohm seals. Moderate seal resistances with PO43- salts were transient and unstable. Median resistances calculated from 24 cells per salt pair.
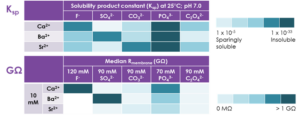
Table 2. Resistances of seals formed using various concentrations of extracellular Ba2+ and intracellular SO42-. Gigaseals only formed with ≥ 3 mM Ba2+ and in the presence of SO42-. Median resistances calculated from 24 or 48 cells per condition.
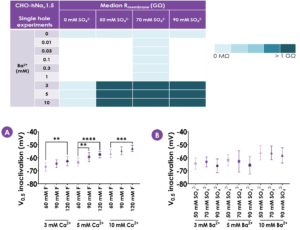

Figure 1. Effects of CaF2 and BaSO4 on hNav1.5 channel biophysics. hNav1.5 V0.5 inactivation with different cation and anion concentrations (mean ± S.D.; N ≥ 11). Increasing concentrations of intracellular F- caused a depolarising shift in V0.5 inactivation (A). In contrast, increasing concentrations of SO42- had no effect on hNav1.5 V0.5 inactivation (B). One-way ANOVAs conducted within each cation group followed by Tukey’s Honestly Significant Difference post-hoc tests: ** = p < .01; *** = p < .001; **** = p < .0001.

Figure 2. CaF2 versus BaSO4 – hNav1.5 pharmacology. A) Representative sweep plots (left) and current-time (I-t) plots (right) for hNav1.5 inhibition by amitriptyline. There was no difference in cumulative inhibition of hNav1.5 by increasing concentrations of amitriptyline between CaF2 and BaSO4. B) Screening of a range of inhibitory compounds showed no difference in hNav1.5 pharmacology between CaF2 and BaSO4. Concentration-response curves for amitriptyline against hNav1.5 using CaF2 or BaSO4 as the seal enhancer (mean ± S.D.; N = 12 wells per concentration for CaF2, N = 8 wells per concentration for BaSO4).
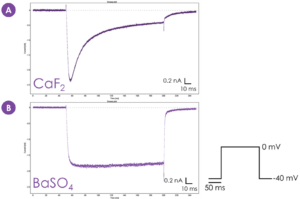
Figure 3. CaF2 versus BaSO4 – hCav1.2 kinetics. hCav1.2 exhibits Ca2+-dependent inactivation when CaF2 is used as the seal enhancer (A). BaSO4 as the seal enhancer (using Ba2+ as a surrogate carrier ion) (B) confers loss of the Ca2+-dependent inactivation of hCav1.2 observed with CaF2. Example sweep plots derived from Sophion Analyzer v9.0.42.
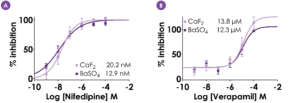
Figure 4. CaF2 versus BaSO4 – hCav1.2 pharmacology. Concentration-response curves for two common inhibitors against hCav1.2, nifedipine (A) and verapamil (B) (CaF2: N = 2‑5 wells per concentration; BaSO4: N = 6-12 wells per concentration). Compound potencies (IC50 values) did not differ between CaF2 and BaSO4. Data displayed as mean ± S.D.






