Lysosomes are acidic vesicles found in eukaryotic cells and ion channels in the lysosomal membrane play a critical role in maintaining the cellular homeostasis. These channels regulate ion flow within the acidic environment of lysosomes, facilitating various cellular processes such as autophagy, endocytosis, and intracellular signalling. Defects in ion channel function can lead to lysosomal dysfunction and, in turn, to an array of diseases. Research in lysosomal ion channels has seen a tremendous increase in popularity over the past decade, due to their involvement in multiple devastating neurodegenerative disorders, lysosomal storage diseases, and cancer.
Research efforts at Metrion have been focusing on the most important lysosomal ion channels: TRPML1, TRPML2, TRPML3, TMEM175 and TPC2, with the aim of developing a suite of validated screening assays - including manual patch-clamp (see video), automated patch-clamp and fluorescence-based techniques (FLIPR) - capable of identifying modulators of these channels.
TRPML1 is a member of the TRP (Transient Receptor Potential) channel family, expressed in the lysosomal membrane. Its principal role is to maintain lysosomal function by regulating calcium and other ion fluxes. TRPML1 is involved in the process of autophagy, the degradation and recycling of cellular components (Scotto Rosato, 2019). Mutations in the TRPML1 gene lead to Mucolipidosis type IV (MLIV), a neurodegenerative disorder characterised by motor impairment, mental retardation, retinal degeneration and anaemia (Dong, 2024). Research into TRPML1 is vital for understanding lysosomal storage diseases and developing potential therapeutic interventions for MLIV. Metrion offers a selection of validated assays capable of confidently identifying modulators of the lysosomal TRPML1 channel. We use a TRPML1 variant (TRPML1-4A) which enables the channel to express at the plasma membrane. As such, channel behaviour can be successfully characterised by whole-cell manual and automated (Qube 384) patch-clamp techniques, and fluorescence-based (FLIPR) assays. Read our full case study here: Development of TRPML1-4A assays across manual, automated patch-clamp, and fluorescence-based platforms. We also offer validated and customisable manual patch-clamp recordings from lysosomes utilising our cell line expressing the wild-type TRPML1 channel.
TRPML2 is another member of the TRPML subfamily and shares structural similarities with TRPML1. However, its functional role remains less understood compared to its counterpart. Its mechanosensitivity, and involvement in promoting recycling in immune cells makes it a promising target for future research (Chen et al, 2020).
TRPML3 is expressed in a variety of tissues, including the inner ear, retina, melanocytes, lungs and kidneys. Unlike TRPML1 and 2, TRPML3 is more active at less acidic pH, making it more likely that it is functionally active in early and recycling endosomes (Spix et al, 2020). Loss or mutations in TRPML3 have been associated with retinal problems, blindness, and deafness (Grimm et al, 2014). There is also emerging evidence for the involvement of TRPML3 in cancer (Santoni et al, 2020). The study of TRPML3 is important for a deeper understanding of a range of associated diseases and identifying new therapies.
TMEM175 operates as a potassium, as well as proton leak channel, and by doing so, it plays an essential role in maintaining an acidic environment inside the lysosome. TMEM175 mutations have been associated with Parkinson's disease, where impaired lysosomal function leads to the accumulation of toxic proteins, a characteristic of neurodegenerative diseases (Wu et al, 2023). Understanding the role of TMEM175 in neuronal health and disease could provide new insights into potential therapeutic strategies for Parkinson's and other neurodegenerative disorders.
TPC2 is a member of the two-pore channel family, primarily located in the lysosomal membrane. The channel regulates calcium release from lysosomes, a process critical for cellular signalling, membrane trafficking, and autophagy (García‐Rúa et al, 2016). Dysregulation of TPC2 has been linked to cardiovascular diseases and neurodegenerative disorders, making it an important target for research focused on disease prevention and treatment (Patel and Kilpatrick, 2018).
Learn more about Metrion’s endo-lysosomal patch clamp assay:
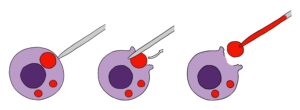
Figure 1: Lysosomal patch clamp technique.
Lysosomes are acidic vesicles found in eukaryotic cells and ion channels in the lysosomal membrane play a critical role in maintaining the cellular homeostasis. These channels regulate ion flow within the acidic environment of lysosomes, facilitating various cellular processes such as autophagy, endocytosis, and intracellular signalling. Defects in ion channel function can lead to lysosomal dysfunction and, in turn, to an array of diseases. Research in lysosomal ion channels has seen a tremendous increase in popularity over the past decade, due to their involvement in multiple devastating neurodegenerative disorders, lysosomal storage diseases, and cancer.
Research efforts at Metrion have been focusing on the most important lysosomal ion channels: TRPML1, TRPML2, TRPML3, TMEM175 and TPC2, with the aim of developing a suite of validated screening assays - including manual patch-clamp (see video), automated patch-clamp and fluorescence-based techniques (FLIPR) - capable of identifying modulators of these channels.
TRPML1 is a member of the TRP (Transient Receptor Potential) channel family, expressed in the lysosomal membrane. Its principal role is to maintain lysosomal function by regulating calcium and other ion fluxes. TRPML1 is involved in the process of autophagy, the degradation and recycling of cellular components (Scotto Rosato, 2019). Mutations in the TRPML1 gene lead to Mucolipidosis type IV (MLIV), a neurodegenerative disorder characterised by motor impairment, mental retardation, retinal degeneration and anaemia (Dong, 2024). Research into TRPML1 is vital for understanding lysosomal storage diseases and developing potential therapeutic interventions for MLIV. Metrion offers a selection of validated assays capable of confidently identifying modulators of the lysosomal TRPML1 channel. We use a TRPML1 variant (TRPML1-4A) which enables the channel to express at the plasma membrane. As such, channel behaviour can be successfully characterised by whole-cell manual and automated (Qube 384) patch-clamp techniques, and fluorescence-based (FLIPR) assays. Read our full case study here: Development of TRPML1-4A assays across manual, automated patch-clamp, and fluorescence-based platforms. We also offer validated and customisable manual patch-clamp recordings from lysosomes utilising our cell line expressing the wild-type TRPML1 channel.
TRPML2 is another member of the TRPML subfamily and shares structural similarities with TRPML1. However, its functional role remains less understood compared to its counterpart. Its mechanosensitivity, and involvement in promoting recycling in immune cells makes it a promising target for future research (Chen et al, 2020).
TRPML3 is expressed in a variety of tissues, including the inner ear, retina, melanocytes, lungs and kidneys. Unlike TRPML1 and 2, TRPML3 is more active at less acidic pH, making it more likely that it is functionally active in early and recycling endosomes (Spix et al, 2020). Loss or mutations in TRPML3 have been associated with retinal problems, blindness, and deafness (Grimm et al, 2014). There is also emerging evidence for the involvement of TRPML3 in cancer (Santoni et al, 2020). The study of TRPML3 is important for a deeper understanding of a range of associated diseases and identifying new therapies.
TMEM175 operates as a potassium, as well as proton leak channel, and by doing so, it plays an essential role in maintaining an acidic environment inside the lysosome. TMEM175 mutations have been associated with Parkinson's disease, where impaired lysosomal function leads to the accumulation of toxic proteins, a characteristic of neurodegenerative diseases (Wu et al, 2023). Understanding the role of TMEM175 in neuronal health and disease could provide new insights into potential therapeutic strategies for Parkinson's and other neurodegenerative disorders.
TPC2 is a member of the two-pore channel family, primarily located in the lysosomal membrane. The channel regulates calcium release from lysosomes, a process critical for cellular signalling, membrane trafficking, and autophagy (García‐Rúa et al, 2016). Dysregulation of TPC2 has been linked to cardiovascular diseases and neurodegenerative disorders, making it an important target for research focused on disease prevention and treatment (Patel and Kilpatrick, 2018).
Learn more about Metrion’s endo-lysosomal patch clamp assay:

Figure 1: Lysosomal patch clamp technique.
Lysosomes are acidic vesicles found in eukaryotic cells and ion channels in the lysosomal membrane play a critical role in maintaining the cellular homeostasis. These channels regulate ion flow within the acidic environment of lysosomes, facilitating various cellular processes such as autophagy, endocytosis, and intracellular signalling. Defects in ion channel function can lead to lysosomal dysfunction and, in turn, to an array of diseases. Research in lysosomal ion channels has seen a tremendous increase in popularity over the past decade, due to their involvement in multiple devastating neurodegenerative disorders, lysosomal storage diseases, and cancer.
Research efforts at Metrion have been focusing on the most important lysosomal ion channels: TRPML1, TRPML2, TRPML3, TMEM175 and TPC2, with the aim of developing a suite of validated screening assays - including manual patch-clamp (see video), automated patch-clamp and fluorescence-based techniques (FLIPR) - capable of identifying modulators of these channels.
TRPML1 is a member of the TRP (Transient Receptor Potential) channel family, expressed in the lysosomal membrane. Its principal role is to maintain lysosomal function by regulating calcium and other ion fluxes. TRPML1 is involved in the process of autophagy, the degradation and recycling of cellular components (Scotto Rosato, 2019). Mutations in the TRPML1 gene lead to Mucolipidosis type IV (MLIV), a neurodegenerative disorder characterised by motor impairment, mental retardation, retinal degeneration and anaemia (Dong, 2024). Research into TRPML1 is vital for understanding lysosomal storage diseases and developing potential therapeutic interventions for MLIV. Metrion offers a selection of validated assays capable of confidently identifying modulators of the lysosomal TRPML1 channel. We use a TRPML1 variant (TRPML1-4A) which enables the channel to express at the plasma membrane. As such, channel behaviour can be successfully characterised by whole-cell manual and automated (Qube 384) patch-clamp techniques, and fluorescence-based (FLIPR) assays. Read our full case study here: Development of TRPML1-4A assays across manual, automated patch-clamp, and fluorescence-based platforms. We also offer validated and customisable manual patch-clamp recordings from lysosomes utilising our cell line expressing the wild-type TRPML1 channel.
TRPML2 is another member of the TRPML subfamily and shares structural similarities with TRPML1. However, its functional role remains less understood compared to its counterpart. Its mechanosensitivity, and involvement in promoting recycling in immune cells makes it a promising target for future research (Chen et al, 2020).
TRPML3 is expressed in a variety of tissues, including the inner ear, retina, melanocytes, lungs and kidneys. Unlike TRPML1 and 2, TRPML3 is more active at less acidic pH, making it more likely that it is functionally active in early and recycling endosomes (Spix et al, 2020). Loss or mutations in TRPML3 have been associated with retinal problems, blindness, and deafness (Grimm et al, 2014). There is also emerging evidence for the involvement of TRPML3 in cancer (Santoni et al, 2020). The study of TRPML3 is important for a deeper understanding of a range of associated diseases and identifying new therapies.
TMEM175 operates as a potassium, as well as proton leak channel, and by doing so, it plays an essential role in maintaining an acidic environment inside the lysosome. TMEM175 mutations have been associated with Parkinson's disease, where impaired lysosomal function leads to the accumulation of toxic proteins, a characteristic of neurodegenerative diseases (Wu et al, 2023). Understanding the role of TMEM175 in neuronal health and disease could provide new insights into potential therapeutic strategies for Parkinson's and other neurodegenerative disorders.
TPC2 is a member of the two-pore channel family, primarily located in the lysosomal membrane. The channel regulates calcium release from lysosomes, a process critical for cellular signalling, membrane trafficking, and autophagy (García‐Rúa et al, 2016). Dysregulation of TPC2 has been linked to cardiovascular diseases and neurodegenerative disorders, making it an important target for research focused on disease prevention and treatment (Patel and Kilpatrick, 2018).
Learn more about Metrion’s endo-lysosomal patch clamp assay:

Figure 1: Lysosomal patch clamp technique.