We were invited by European Biopharmaceutical Review to discuss how advancements in ion channel drug discovery are developing research in the field of pain management.
The Q&A with Dr Eddy Stevens, CSO, and Dr Tony Rush, Director of Neuroscience at Metrion, follows and can also be seen in the online Spring issue of European Biopharmaceutical Review on pages 38-40 (from page 78 of pdf).
Drug discovery in pain management is critical due to the high prevalence of chronic pain conditions and the limitations of existing treatments, such as opioids and gabapentinoids, which carry risks of addiction and side effects. Many current analgesics lack specificity and fail to address the underlying mechanisms of pain. A key focus of current drug discovery programs, in a quest to develop safer and more efficacious analgesics, is targeting peripheral pain mechanisms.
Ion channels play a key role in pain perception by regulating neuronal excitability and pain signal transmission. Voltage-gated sodium channels (NaV1.7, NaV1.8, NaV1.9) which underlie action potential electrogenesis in nociceptors are key targets, exemplified by genetic associations with clinical pain conditions (for NaV1.7 and NaV1.9)1. Calcium channels control synaptic release from nociceptors within the dorsal horn of the spinal cord (where gabapentinoids act on CaV2.2 accessory subunit a2d1). Potassium channels (e.g. Kv7, TREK1 and TRESK) act as ‘brakes on excitability’ by stabilising neuronal activity. Acid-sensing ion channels (ASICs) contribute to pain caused by tissue acidosis.
With greater awareness of the issues with current pain medications (such as opioids) pain management research has been striving to develop safer and more effective treatments. Some innovations in drug delivery systems, such as transdermal patches, have improved the administration and efficacy of analgesics. In an exciting recent development, Vertex announced the approval of a NaV1.8 blocker, Suzetrigine, which helps treat acute pain and is non-addictive.
In summary, the past five years have witnessed substantial progress in pain management research, with ion channel studies contributing significantly to the development of innovative and safer therapeutic options.
Advancing pain management research, particularly in ion channel drug discovery, requires improvements in technology, methodology, and funding. High-throughput screening (HTS) advancements, such as automated patch-clamp systems, have certainly accelerated the identification of ion channel modulators. Research on additional targets, including NaV1.9, which has been difficult to research, would also help the pain discovery field.
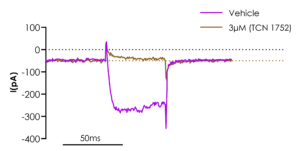
Figure 1. Stable NaV1.9 cell line manual patch clamp recordings showing inhibition with the sodium channel blocker, TC-N 1752. The published IC50 value for this blocker is 1.6 uM2 which is consistent with the data shown in the figure.
Developing more physiologically relevant models, such as iPSC-derived sensory neurons, would enhance understanding of ion channel activity in pain conditions. AI-driven drug discovery and in silico screening could significantly improve hit-to-lead success rates, allowing for faster identification of promising compounds targeting NaV1.8, NaV1.9 and KV7.2/Kv7.3.
Increased investment in non-opioid pain research is essential, with greater government and private funding encouraging pharmaceutical innovation. Precision medicine, supported by genetic profiling, could help tailor treatments for specific patient populations, such as those with NaV1.7/NaV1.9 mutations linked to congenital pain disorders.
New drug modalities, including monoclonal antibodies, gene therapy, and peptide-based NaV1.7 inhibitors, may offer promising alternatives with the potential of fewer side effects compared to small molecule approaches.
By integrating cutting-edge HTS technologies, AI-driven discovery, genetic insights, and novel drug modalities, ion channel research can lead to the next generation of pain therapeutics.
Raising awareness about pain and its management requires a multifaceted approach that educates the public, healthcare providers, and policymakers on the complexity of pain and the latest scientific advancements, including ion channel research.
One key strategy is public education campaigns that explain the biological basis of pain, emphasising that pain is not just a symptom but a complex neurological process. Highlighting the role of ion channels in peripheral mechanisms of pain (controlling pain transmission into the spinal cord) can help society understand how pain arises and why targeted treatments are necessary. Media and advocacy efforts can drive awareness by featuring stories of patients with chronic pain and explaining new scientific breakthroughs in ion channel-based therapeutics. Encouraging coverage of non-opioid drug development in pain research, including advances made in ion channel research, could help shift the conversation toward innovative solutions.
Finally, policy initiatives supporting research into therapies focused on peripheral pain mechanisms (e.g. targeting ion channels) can promote funding and regulatory changes that prioritise non-opioid drug discovery, leading to better, safer treatments for patients. Improved treatments would also have significant economic and societal benefits, where better individual health allows a greater contribution to both.
We were invited by European Biopharmaceutical Review to discuss how advancements in ion channel drug discovery are developing research in the field of pain management.
The Q&A with Dr Eddy Stevens, CSO, and Dr Tony Rush, Director of Neuroscience at Metrion, follows and can also be seen in the online Spring issue of European Biopharmaceutical Review on pages 38-40 (from page 78 of pdf).
Drug discovery in pain management is critical due to the high prevalence of chronic pain conditions and the limitations of existing treatments, such as opioids and gabapentinoids, which carry risks of addiction and side effects. Many current analgesics lack specificity and fail to address the underlying mechanisms of pain. A key focus of current drug discovery programs, in a quest to develop safer and more efficacious analgesics, is targeting peripheral pain mechanisms.
Ion channels play a key role in pain perception by regulating neuronal excitability and pain signal transmission. Voltage-gated sodium channels (NaV1.7, NaV1.8, NaV1.9) which underlie action potential electrogenesis in nociceptors are key targets, exemplified by genetic associations with clinical pain conditions (for NaV1.7 and NaV1.9)1. Calcium channels control synaptic release from nociceptors within the dorsal horn of the spinal cord (where gabapentinoids act on CaV2.2 accessory subunit a2d1). Potassium channels (e.g. Kv7, TREK1 and TRESK) act as ‘brakes on excitability’ by stabilising neuronal activity. Acid-sensing ion channels (ASICs) contribute to pain caused by tissue acidosis.
With greater awareness of the issues with current pain medications (such as opioids) pain management research has been striving to develop safer and more effective treatments. Some innovations in drug delivery systems, such as transdermal patches, have improved the administration and efficacy of analgesics. In an exciting recent development, Vertex announced the approval of a NaV1.8 blocker, Suzetrigine, which helps treat acute pain and is non-addictive.
In summary, the past five years have witnessed substantial progress in pain management research, with ion channel studies contributing significantly to the development of innovative and safer therapeutic options.
Advancing pain management research, particularly in ion channel drug discovery, requires improvements in technology, methodology, and funding. High-throughput screening (HTS) advancements, such as automated patch-clamp systems, have certainly accelerated the identification of ion channel modulators. Research on additional targets, including NaV1.9, which has been difficult to research, would also help the pain discovery field.

Figure 1. Stable NaV1.9 cell line manual patch clamp recordings showing inhibition with the sodium channel blocker, TC-N 1752. The published IC50 value for this blocker is 1.6 uM2 which is consistent with the data shown in the figure.
Developing more physiologically relevant models, such as iPSC-derived sensory neurons, would enhance understanding of ion channel activity in pain conditions. AI-driven drug discovery and in silico screening could significantly improve hit-to-lead success rates, allowing for faster identification of promising compounds targeting NaV1.8, NaV1.9 and KV7.2/Kv7.3.
Increased investment in non-opioid pain research is essential, with greater government and private funding encouraging pharmaceutical innovation. Precision medicine, supported by genetic profiling, could help tailor treatments for specific patient populations, such as those with NaV1.7/NaV1.9 mutations linked to congenital pain disorders.
New drug modalities, including monoclonal antibodies, gene therapy, and peptide-based NaV1.7 inhibitors, may offer promising alternatives with the potential of fewer side effects compared to small molecule approaches.
By integrating cutting-edge HTS technologies, AI-driven discovery, genetic insights, and novel drug modalities, ion channel research can lead to the next generation of pain therapeutics.
Raising awareness about pain and its management requires a multifaceted approach that educates the public, healthcare providers, and policymakers on the complexity of pain and the latest scientific advancements, including ion channel research.
One key strategy is public education campaigns that explain the biological basis of pain, emphasising that pain is not just a symptom but a complex neurological process. Highlighting the role of ion channels in peripheral mechanisms of pain (controlling pain transmission into the spinal cord) can help society understand how pain arises and why targeted treatments are necessary. Media and advocacy efforts can drive awareness by featuring stories of patients with chronic pain and explaining new scientific breakthroughs in ion channel-based therapeutics. Encouraging coverage of non-opioid drug development in pain research, including advances made in ion channel research, could help shift the conversation toward innovative solutions.
Finally, policy initiatives supporting research into therapies focused on peripheral pain mechanisms (e.g. targeting ion channels) can promote funding and regulatory changes that prioritise non-opioid drug discovery, leading to better, safer treatments for patients. Improved treatments would also have significant economic and societal benefits, where better individual health allows a greater contribution to both.
We were invited by European Biopharmaceutical Review to discuss how advancements in ion channel drug discovery are developing research in the field of pain management.
The Q&A with Dr Eddy Stevens, CSO, and Dr Tony Rush, Director of Neuroscience at Metrion, follows and can also be seen in the online Spring issue of European Biopharmaceutical Review on pages 38-40 (from page 78 of pdf).
Drug discovery in pain management is critical due to the high prevalence of chronic pain conditions and the limitations of existing treatments, such as opioids and gabapentinoids, which carry risks of addiction and side effects. Many current analgesics lack specificity and fail to address the underlying mechanisms of pain. A key focus of current drug discovery programs, in a quest to develop safer and more efficacious analgesics, is targeting peripheral pain mechanisms.
Ion channels play a key role in pain perception by regulating neuronal excitability and pain signal transmission. Voltage-gated sodium channels (NaV1.7, NaV1.8, NaV1.9) which underlie action potential electrogenesis in nociceptors are key targets, exemplified by genetic associations with clinical pain conditions (for NaV1.7 and NaV1.9)1. Calcium channels control synaptic release from nociceptors within the dorsal horn of the spinal cord (where gabapentinoids act on CaV2.2 accessory subunit a2d1). Potassium channels (e.g. Kv7, TREK1 and TRESK) act as ‘brakes on excitability’ by stabilising neuronal activity. Acid-sensing ion channels (ASICs) contribute to pain caused by tissue acidosis.
With greater awareness of the issues with current pain medications (such as opioids) pain management research has been striving to develop safer and more effective treatments. Some innovations in drug delivery systems, such as transdermal patches, have improved the administration and efficacy of analgesics. In an exciting recent development, Vertex announced the approval of a NaV1.8 blocker, Suzetrigine, which helps treat acute pain and is non-addictive.
In summary, the past five years have witnessed substantial progress in pain management research, with ion channel studies contributing significantly to the development of innovative and safer therapeutic options.
Advancing pain management research, particularly in ion channel drug discovery, requires improvements in technology, methodology, and funding. High-throughput screening (HTS) advancements, such as automated patch-clamp systems, have certainly accelerated the identification of ion channel modulators. Research on additional targets, including NaV1.9, which has been difficult to research, would also help the pain discovery field.

Figure 1. Stable NaV1.9 cell line manual patch clamp recordings showing inhibition with the sodium channel blocker, TC-N 1752. The published IC50 value for this blocker is 1.6 uM2 which is consistent with the data shown in the figure.
Developing more physiologically relevant models, such as iPSC-derived sensory neurons, would enhance understanding of ion channel activity in pain conditions. AI-driven drug discovery and in silico screening could significantly improve hit-to-lead success rates, allowing for faster identification of promising compounds targeting NaV1.8, NaV1.9 and KV7.2/Kv7.3.
Increased investment in non-opioid pain research is essential, with greater government and private funding encouraging pharmaceutical innovation. Precision medicine, supported by genetic profiling, could help tailor treatments for specific patient populations, such as those with NaV1.7/NaV1.9 mutations linked to congenital pain disorders.
New drug modalities, including monoclonal antibodies, gene therapy, and peptide-based NaV1.7 inhibitors, may offer promising alternatives with the potential of fewer side effects compared to small molecule approaches.
By integrating cutting-edge HTS technologies, AI-driven discovery, genetic insights, and novel drug modalities, ion channel research can lead to the next generation of pain therapeutics.
Raising awareness about pain and its management requires a multifaceted approach that educates the public, healthcare providers, and policymakers on the complexity of pain and the latest scientific advancements, including ion channel research.
One key strategy is public education campaigns that explain the biological basis of pain, emphasising that pain is not just a symptom but a complex neurological process. Highlighting the role of ion channels in peripheral mechanisms of pain (controlling pain transmission into the spinal cord) can help society understand how pain arises and why targeted treatments are necessary. Media and advocacy efforts can drive awareness by featuring stories of patients with chronic pain and explaining new scientific breakthroughs in ion channel-based therapeutics. Encouraging coverage of non-opioid drug development in pain research, including advances made in ion channel research, could help shift the conversation toward innovative solutions.
Finally, policy initiatives supporting research into therapies focused on peripheral pain mechanisms (e.g. targeting ion channels) can promote funding and regulatory changes that prioritise non-opioid drug discovery, leading to better, safer treatments for patients. Improved treatments would also have significant economic and societal benefits, where better individual health allows a greater contribution to both.