The Metrion Biosciences team recently produced an application note reporting the successful validation of the ‘Milnes’ voltage protocol for hERG screening using a QPatch 48 automated patch clamp (APC) assay platform. First reported in 2010 using the conventional whole cell patch clamp technique (Milnes et al., 2010, J. Pharmacol. Toxicol. Methods, 61(2), 178-191), this assay protocol has proven to be a significant challenge for transfer to automated electrophysiology systems and implementation by the Metrion Biosciences team further strengthens our portfolio of cardiac safety assays.
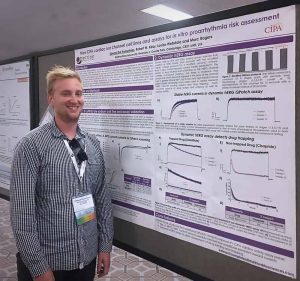
Reliable and cost-effective cardiac safety screening data is fundamental to the discovery and development of safe and efficacious new drugs. With this in mind, the Food and Drug Administration’s Comprehensive in-vitro Proarrythmia Assay (CiPA) initiative was set up to develop and validate an improved process for predicting arrhythmogenic risk at an early stage in discovery research. This involved broadening the in-vitro cardiac ion channel screening panel and the use of in-silico models of the human cardiac action potential using data obtained from the expanded ion channel panel. Inclusion of data assessing the kinetics of hERG block by drugs using the ‘Milnes’ voltage protocol to a modified ‘dynamic’ O’Hara-Rudy in-silico model, is regarded as a route to improved predictions of cardiac liability. Prior to release of our application note there was no publicly available automated electrophysiology implementation data for this type of assay, with conventional whole cell patch clamp being the method widely used to measure hERG blocking kinetics and drug trapping.
Metrion’s kinetic hERG assay application note reviews our implementation of the Milnes protocol to generate data for four drugs with varying kinetics and trapping capacities (dofetilide, terfenadine, verapamil and cisapride) on QPatch 48. The results and methods are discussed within the full version of the document, with our underlying conclusion that it is possible to use an APC platform (QPatch 48) to produce high quality data in agreement with that of gold-standard manual patch clamp studies. Such data produced using APC platforms will facilitate improvements towards optimised cardiac safety testing and therefore streamlining the journey to safer and more efficacious medicines.
The full application note can be found here on the Metrion Biosciences website. You can contact our cardiac safety experts or request further information via info@metrionbioscienes.com.
Metrion Biosciences’ Edward Humphries first presented data contained within the application note at the 2018 Sophion European Users Meeting. His research was supported by Metrion team members Rob Kirby, John Ridley, Warren Miller, Louise Webdale and Marc Rogers.
We would also like to thank Thomas Binzer and the Sophion Biosciences team for their support and involvement in making this application note possible.
The Metrion Biosciences team recently produced an application note reporting the successful validation of the ‘Milnes’ voltage protocol for hERG screening using a QPatch 48 automated patch clamp (APC) assay platform. First reported in 2010 using the conventional whole cell patch clamp technique (Milnes et al., 2010, J. Pharmacol. Toxicol. Methods, 61(2), 178-191), this assay protocol has proven to be a significant challenge for transfer to automated electrophysiology systems and implementation by the Metrion Biosciences team further strengthens our portfolio of cardiac safety assays.

Reliable and cost-effective cardiac safety screening data is fundamental to the discovery and development of safe and efficacious new drugs. With this in mind, the Food and Drug Administration’s Comprehensive in-vitro Proarrythmia Assay (CiPA) initiative was set up to develop and validate an improved process for predicting arrhythmogenic risk at an early stage in discovery research. This involved broadening the in-vitro cardiac ion channel screening panel and the use of in-silico models of the human cardiac action potential using data obtained from the expanded ion channel panel. Inclusion of data assessing the kinetics of hERG block by drugs using the ‘Milnes’ voltage protocol to a modified ‘dynamic’ O’Hara-Rudy in-silico model, is regarded as a route to improved predictions of cardiac liability. Prior to release of our application note there was no publicly available automated electrophysiology implementation data for this type of assay, with conventional whole cell patch clamp being the method widely used to measure hERG blocking kinetics and drug trapping.
Metrion’s kinetic hERG assay application note reviews our implementation of the Milnes protocol to generate data for four drugs with varying kinetics and trapping capacities (dofetilide, terfenadine, verapamil and cisapride) on QPatch 48. The results and methods are discussed within the full version of the document, with our underlying conclusion that it is possible to use an APC platform (QPatch 48) to produce high quality data in agreement with that of gold-standard manual patch clamp studies. Such data produced using APC platforms will facilitate improvements towards optimised cardiac safety testing and therefore streamlining the journey to safer and more efficacious medicines.
The full application note can be found here on the Metrion Biosciences website. You can contact our cardiac safety experts or request further information via info@metrionbioscienes.com.
Metrion Biosciences’ Edward Humphries first presented data contained within the application note at the 2018 Sophion European Users Meeting. His research was supported by Metrion team members Rob Kirby, John Ridley, Warren Miller, Louise Webdale and Marc Rogers.
We would also like to thank Thomas Binzer and the Sophion Biosciences team for their support and involvement in making this application note possible.
The Metrion Biosciences team recently produced an application note reporting the successful validation of the ‘Milnes’ voltage protocol for hERG screening using a QPatch 48 automated patch clamp (APC) assay platform. First reported in 2010 using the conventional whole cell patch clamp technique (Milnes et al., 2010, J. Pharmacol. Toxicol. Methods, 61(2), 178-191), this assay protocol has proven to be a significant challenge for transfer to automated electrophysiology systems and implementation by the Metrion Biosciences team further strengthens our portfolio of cardiac safety assays.

Reliable and cost-effective cardiac safety screening data is fundamental to the discovery and development of safe and efficacious new drugs. With this in mind, the Food and Drug Administration’s Comprehensive in-vitro Proarrythmia Assay (CiPA) initiative was set up to develop and validate an improved process for predicting arrhythmogenic risk at an early stage in discovery research. This involved broadening the in-vitro cardiac ion channel screening panel and the use of in-silico models of the human cardiac action potential using data obtained from the expanded ion channel panel. Inclusion of data assessing the kinetics of hERG block by drugs using the ‘Milnes’ voltage protocol to a modified ‘dynamic’ O’Hara-Rudy in-silico model, is regarded as a route to improved predictions of cardiac liability. Prior to release of our application note there was no publicly available automated electrophysiology implementation data for this type of assay, with conventional whole cell patch clamp being the method widely used to measure hERG blocking kinetics and drug trapping.
Metrion’s kinetic hERG assay application note reviews our implementation of the Milnes protocol to generate data for four drugs with varying kinetics and trapping capacities (dofetilide, terfenadine, verapamil and cisapride) on QPatch 48. The results and methods are discussed within the full version of the document, with our underlying conclusion that it is possible to use an APC platform (QPatch 48) to produce high quality data in agreement with that of gold-standard manual patch clamp studies. Such data produced using APC platforms will facilitate improvements towards optimised cardiac safety testing and therefore streamlining the journey to safer and more efficacious medicines.
The full application note can be found here on the Metrion Biosciences website. You can contact our cardiac safety experts or request further information via info@metrionbioscienes.com.
Metrion Biosciences’ Edward Humphries first presented data contained within the application note at the 2018 Sophion European Users Meeting. His research was supported by Metrion team members Rob Kirby, John Ridley, Warren Miller, Louise Webdale and Marc Rogers.
We would also like to thank Thomas Binzer and the Sophion Biosciences team for their support and involvement in making this application note possible.