Robust high-throughput automated electrophysiology assay using a monoclonal CHO-hNav1.9 cellular reagent suitable for fully supporting a Nav1.9 discovery program.
James Masterson, Sergiy Tokar, Ayesha Jinat, Robert Kirby
Metrion Biosciences Ltd, Granta Centre, Granta Park, Cambridge, CB21 6AL, United Kingdom
Good Laboratory Practice (GLP) hERG testing is a critical component in the preclinical safety evaluation of new chemical entities. The human ether-à-go-go-related gene (hERG) encodes a key potassium ion channel that plays an essential role in the repolarisation phase of the cardiac action potential. Any alterations in the function of these channels can lead to severe cardiac arrhythmias and potentially fatal outcomes. As such, a robust hERG assay is indispensable in predicting cardiotoxicity risk. This publication details the validation of Metrion’s GLP hERG testing assay in strict accordance with the ICH E14/S7B 2022 Q&A best practice guidelines, ensuring that our methods deliver high-quality, reproducible, and transparent data.
The ICH E14/S7B 2022 Q&A guidelines provide comprehensive recommendations on evaluating the effects of preclinical compounds on hERG potassium ion channels. These guidelines ensure that the in vitro hERG assessments are carried out in full GLP compliance, thereby bolstering data integrity and regulatory acceptance. The guidelines recommend specific experimental methods, quality control measures, and reporting formats designed to achieve industry-wide consistency and transparency.
A central tenet of the guidelines is the use of positive controls - ondansetron, moxifloxacin, and dofetilide - to establish defined safety margins. In our study, a hERG assay is deemed negative if the test article’s safety margin exceeds those derived from these controls. This framework is essential to both industry best practice and regulatory compliance.
The cornerstone of our GLP hERG testing protocol is the use of Chinese hamster ovary (CHO) cells stably expressing the hERG1a isoform. These cells are well recognised for their consistent and robust expression of potassium ion channels, making them ideal for electrophysiological studies. The cells were carefully plated onto glass coverslips and maintained at 37 °C for 24 to 48 hours prior to electrophysiological recordings. This incubation period ensures optimal cell adhesion and viability, which is critical for obtaining reliable hERG assay data.
To faithfully mimic the physiological environment during recordings, specialised intracellular and extracellular solutions were prepared:
For a robust hERG assay, positive control compounds must be precisely prepared:
The manual patch clamp technique is considered the gold standard for GLP hERG testing. Our whole-cell voltage-clamp experiments were conducted using a HEKA EPC10 patch clamp amplifier paired with PatchMaster Pro (version V2.73) software. Recordings were carried out at 36 ± 1 °C to closely replicate human physiological conditions. Key details include:
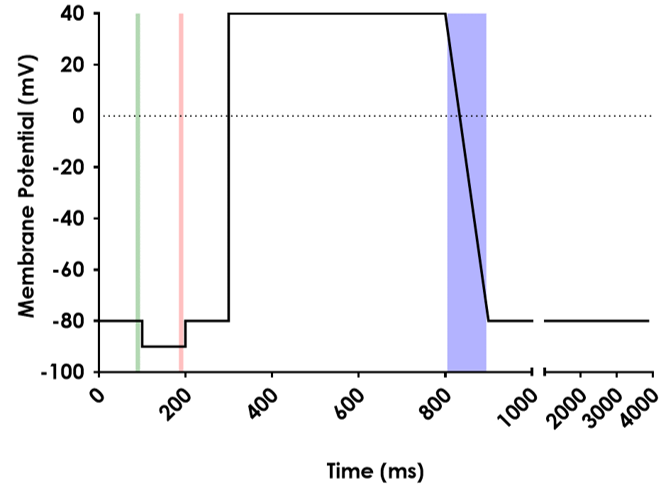
Figure 1. The voltage protocol that was used to elicit hERG current. Shaded areas indicate the locations of the cursors used to analyse the holding current (green), the current elicited by a step to -90 mV (red), and the peak current (blue).
Following the establishment of the whole-cell configuration, cells were initially superfused with extracellular solution containing the vehicle (0.1% DMSO) to allow the current to stabilise during the control period. After stabilisation, compounds were applied using either a two-point assay format (for ondansetron and moxifloxacin) or a single-point assay format (for dofetilide). Each experiment was concluded with the application of 1 µM E-4031, which permitted the subtraction of leak and endogenous currents from the recorded data.
Quality control was rigorously maintained by continuously monitoring the peak current amplitude, input resistance, series resistance, and holding current over time. Cells exhibiting significant fluctuations were discarded to ensure that only high-quality data were used in the analysis. Additionally, samples of the compounds were collected after each experiment to verify concentration accuracy, further enhancing the reliability of our hERG assay.
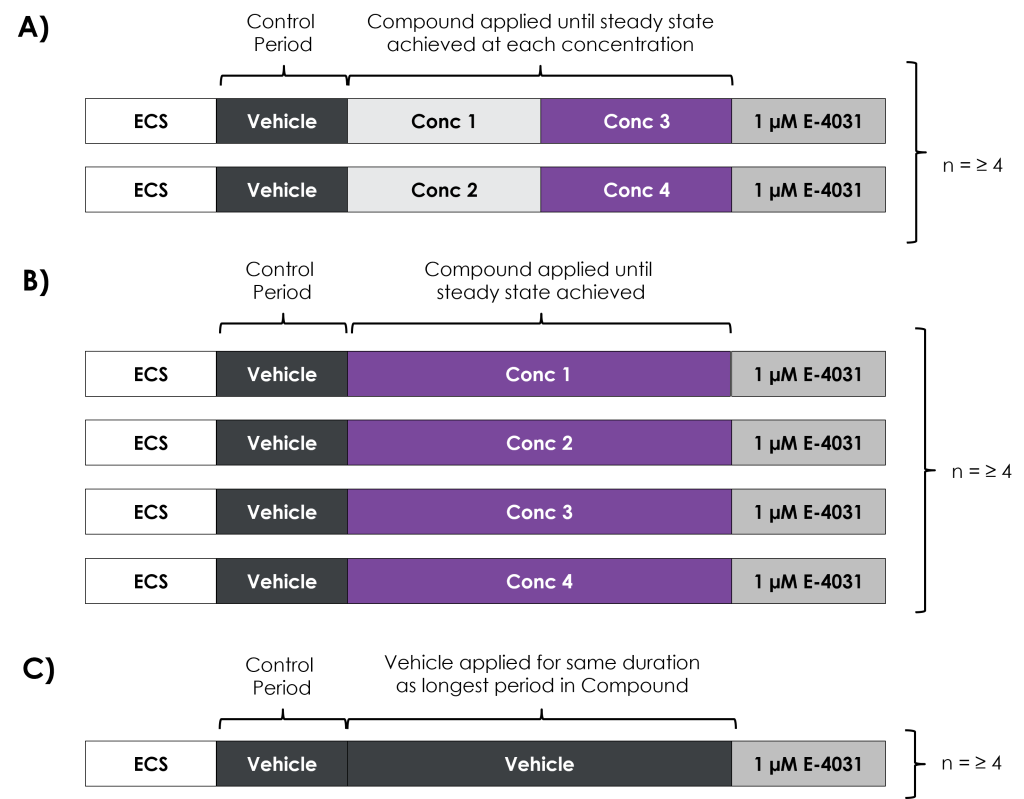
Figure 2. Paradigms used for compound application, including two-point assay formats for ondansetron and moxifloxacin (Figure 2A) and a single-point assay format for dofetilide (Figure 2B), as well as vehicle controls (Figure 2C).
Data analysis in GLP hERG testing is critical for determining the safety margins of test compounds. In our study, the percentage inhibition of the peak current amplitude was calculated using values derived after subtracting the effects of 1 µM E-4031. The mean inhibition data (± standard deviation) were plotted against the logarithm of the compound concentrations, and the data were fitted using a four-parameter logistic equation. This analysis allowed us to derive in-house IC50 values for each compound, complete with 95% confidence intervals.
This robust data analysis protocol ensures that the hERG assay is not only reproducible but also adheres strictly to the quality control measures dictated by the ICH E14/S7B guidelines. The successful validation of the assay using ondansetron, moxifloxacin, and dofetilide confirms that our GLP hERG testing protocol reliably measures the impact on potassium ion channels, offering an indispensable tool in the preclinical safety assessment of investigational drugs.
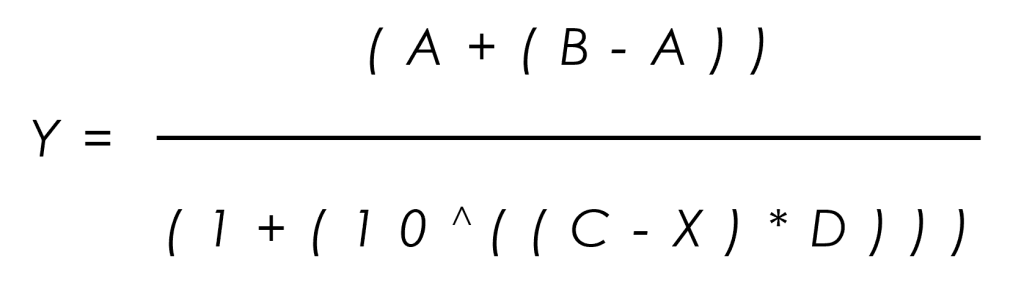
Our GLP hERG testing assay produced high-resolution current traces that clearly demonstrate the inhibitory effects of the positive control compounds on hERG channels. These recordings allowed for the precise quantification of the effects on peak current amplitude, input resistance, series resistance, and holding current.
Representative current traces obtained from experiments with ondansetron, moxifloxacin, and dofetilide, alongside vehicle controls are shown in Figure 3. This figure also includes key electrophysiological parameters such as peak current amplitude and input resistance, reinforcing the consistency of our data.
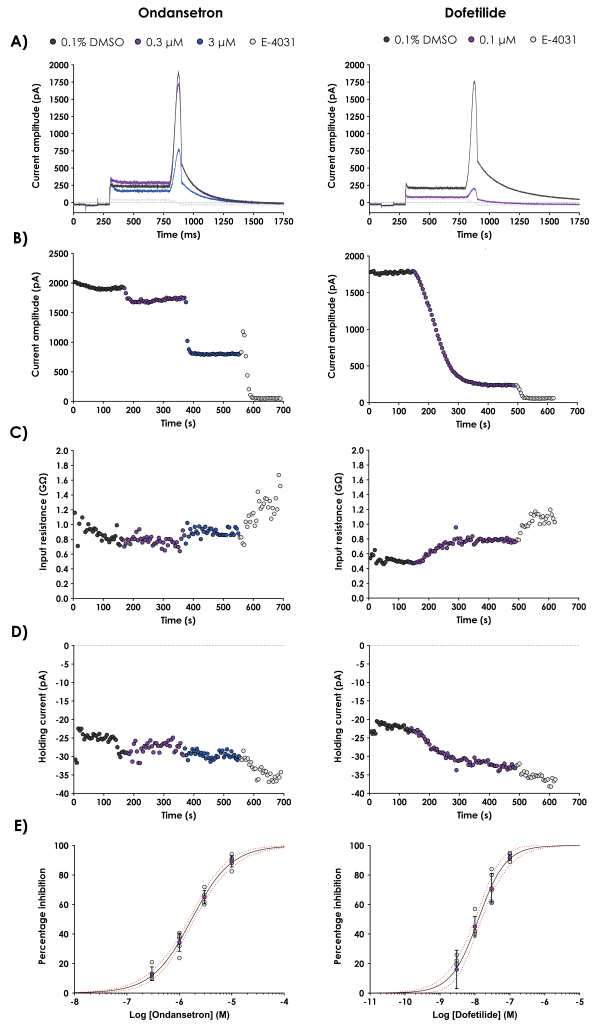
Figure 3. Representative current traces obtained from experiments with ondansetron, moxifloxacin, and dofetilide, alongside vehicle controls.
To further support our findings, we include two tables that summarise critical electrophysiological parameters and IC50 values derived from our GLP hERG testing assay.
Table 1: The mean (± SEM) Peak Current amplitude, Input Resistance, Series Resistance and Holding Current values recorded at the end of each Control Period.
| Ondansetron | Moxifloxacin | Dofetilide | Vehicle | |
| Peak Current (pA) | 1535.3 ± 242.5 | 1257.9 ± 119.1 | 1360.2 ± 170.9 | 1324.1 ± 242.4 |
| Input Resistance (MΩ) | 770 ± 90 | 580 ± 30 | 560 ± 50 | 680 ± 80 |
| Series Resistance (MΩ) | 3.5 ± 2.1 | 4.0 ± 3.0 | 3.2 ± 2.1 | 3.2 ± 1.3 |
| Holding Current (pA) | -32.1 ± 5.5 | -37.1 ± 6.4 | -25.5 ± 3.6 | -18.4 ± 6.9 |
Table 2 compares the IC50 values and 95% confidence intervals reported in the ICH E14/S7B training material with those obtained by Metrion in this study for moxifloxacin, ondansetron, and dofetilide.
| Compound | ICH E14/S7B Training Material IC50 (µM) (95% Confidence Intervals) | Metrion IC50 (µM) (95% Confidence Intervals) |
| Moxifloxacin | 62 (38, 104) | 96.2 (78.6, 117.7) |
| Ondansetron | 1.4 (0.8, 2.6) | 1.72 (1.51, 1.95) |
| Dofetilide | 0.01 (<0.01, 0.02) | 0.012 (0.011, 0.013) |
Consistency with ICH E14/S7B training material
The IC50 values obtained in our study were within a twofold range of those reported in the ICH E14/S7B training material. This consistency confirms that our hERG assay, conducted in full GLP compliance, provides a reliable benchmark for evaluating test articles. The data underscore the importance of using well-established positive controls - ondansetron, moxifloxacin, and dofetilide - in determining the safety margins for potential cardiotoxic compounds.
The validation of our GLP hERG testing assay has significant implications for the pharmaceutical industry. By rigorously adhering to the ICH E14/S7B Q&A best practice guidelines, we have established a robust framework for evaluating the effects of preclinical compounds on potassium ion channels. The detailed electrophysiological data generated by our manual patch clamp technique provide clear insights into the safety profiles of investigational drugs, thereby reducing the risk of drug-induced cardiac arrhythmias.
GLP compliance is not only a regulatory requirement but also a hallmark of quality in preclinical testing. By ensuring that our hERG assay is conducted under GLP conditions, we can guarantee the transparency, consistency, and reliability of our data. This commitment to GLP hERG testing reinforces our position as a leader in the field of ion channel research and supports safer drug development practices.
Looking ahead, there is considerable potential for integrating emerging automated patch clamp systems with traditional manual techniques. Such innovations promise to increase throughput while preserving the high fidelity of electrophysiological recordings. Continued refinement of GLP hERG testing protocols will further enhance our understanding of potassium ion channels, ultimately contributing to the development of safer therapeutic agents.
In summary, our GLP hERG testing assay validation - conducted in line with the ICH E14/S7B 2022 Q&A best practice guidelines - represents a significant advancement in the preclinical assessment of drug safety. Through meticulous cell preparation, optimised recording solutions, precise compound handling, and rigorous data analysis, we have demonstrated that our hERG assay provides reliable and reproducible measurements of potassium ion channel activity.
The successful validation of the assay using positive controls such as ondansetron, moxifloxacin, and dofetilide confirms its robustness and accuracy. This work not only enhances the overall reliability of preclinical testing but also sets a new benchmark for GLP hERG testing within the industry.
For organisations committed to the highest standards of drug safety, our publication provides a comprehensive guide on implementing best practice methodologies. By prioritising quality control and regulatory compliance, we continue to advance the field of potassium ion channel research, ultimately contributing to the development of safer and more effective therapeutic agents.
This detailed report is an essential resource for scientists and regulatory professionals alike, offering valuable insights into the optimisation of hERG assays and GLP hERG testing. Through our unwavering commitment to excellence, Metrion continues to lead the way in preclinical electrophysiological research, ensuring that innovative drugs are developed with the highest safety standards in mind.
Robust high-throughput automated electrophysiology assay using a monoclonal CHO-hNav1.9 cellular reagent suitable for fully supporting a Nav1.9 discovery program.
To overcome seal enhancer limitations, Sophion and Metrion collaborated to determine whether other insoluble salts can act as seal enhancers and how these solution pairs affect the biophysical properties and pharmacology of the investigated ion channels.