How advancements in ion channel drug discovery are developing research in the field of pain management

Unique approach | Critical pain target | Overcoming challenges in studying NaV1.9 | Advantages of automated patch clamp | Why choose Metrion | Beyond NaV1.9 | Apply Metrion's expertise to your NaV1.9 research
Ion channels are a major class of drug targets for modulating pain transmission and controlling chronic pain. The voltage-gated sodium channel, NaV1.9, has been identified as a critical component of pain signalling. While NaV1.9 holds great promise as a non-opioid pain target, the discovery and development of new chemical entities has been hampered by the availability of robust cellular reagents suitable for high-throughput electrophysiology. The global chronic pain treatment market was valued at $88.2 billion in 2023 and is projected to reach $173.4 billion by 2033, growing at a 7.0% CAGR. Neuropathic pain, a major segment of this market, represents around 31.6% of the total.1
Metrion has addressed this challenge with the development of robust stably expressing NaV1.9 cell lines (human and rat) which have been validated on the QPatch 48 and Qube 384 electrophysiology platforms. This enables:
With over a decade of electrophysiology expertise, the NaV1.9 assay, alongside Metrion’s unique combination of ion channel expertise, bespoke assays and pain research services, enables researchers to overcome traditional limitations of NaV1.9 screening, to generate reproducible and decision-ready data.
The business case is clear for organisations looking to rapidly meet project milestones and unlock ROI in pain drug discovery:
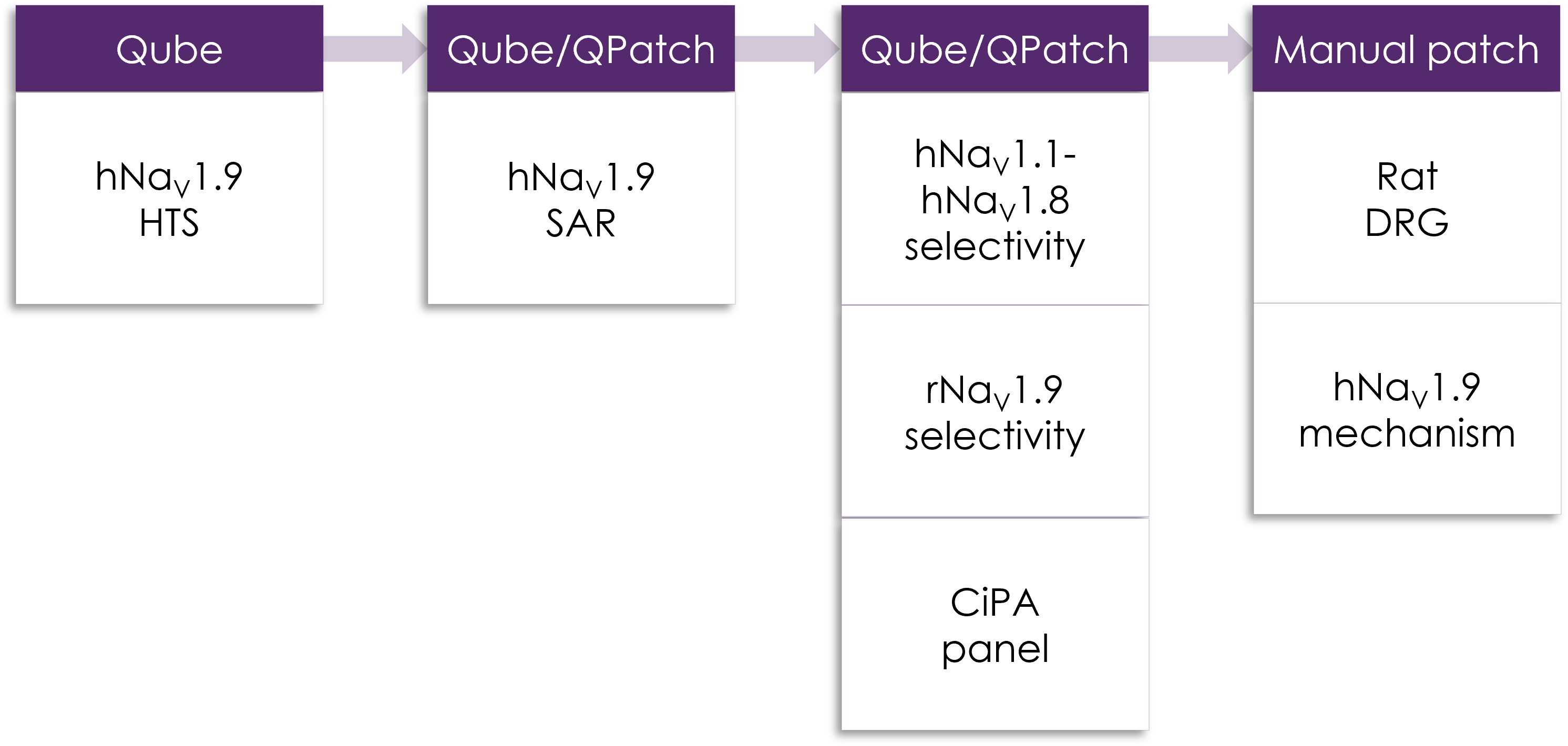
Figure 1. Example of Metrion's NaV1.9 screening cascade.
Alongside our highly experienced electrophysiologists, Metrion’s NaV1.9 drug discovery assays, in combination with other pain research related services, sets the Metrion offering apart from others by combining the following to support our customers’ discovery needs:
Despite its importance in pain research, NaV1.9 remains one of the most difficult sodium channel targets to prosecute due to:
Metrion has overcome these challenges by developing robust NaV1.9 cell lines which have been validated using the QPatch 48 and Qube 384 providing high quality reproducible data.
Our validated, high-throughput NaV1.9 screening solution delivers consistent and accurate results. Key advantages:
These enhancements place Metrion at the forefront of NaV1.9 drug discovery.
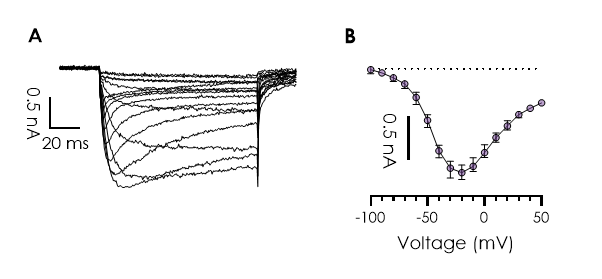
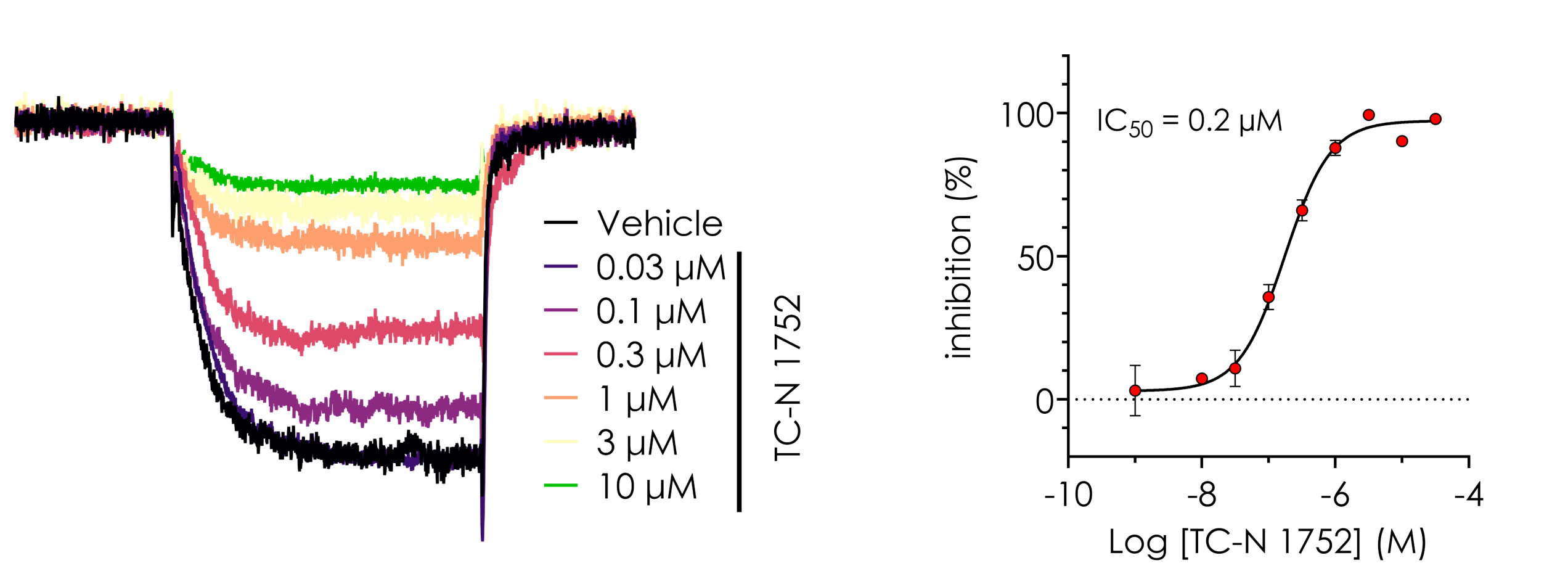
Figure 3. Pharmacological assessment of human NaV1.9. Current traces and concentration response curves for TC-N 1752, obtained using Qube 384.
NaV1.9 is a voltage-gated sodium channel predominantly expressed in peripheral sensory neurons, where it plays a vital role in pain signalling. These channels contribute to the excitability of nociceptive neurons directly influencing pain pathways. The unique biophysical properties of NaV1.9 plays a key role in regulating the threshold for neuronal firing and is seen as an attractive target for non-opioid pain management.
NaV1.9 has been linked to various pain disorders through genetic mutations leading to both chronic pain syndromes and insensitivity to pain. These discoveries further support the significance of NaV1.9 in pain physiology and the importance of this particular sodium channel subtype as a pain target.
Leading the way in NaV1.9 hit finding, Metrion has exceptional expertise in automated electrophysiology platforms, which delivers:
Uniquely positioned as a leader in ion channel drug discovery, Metrion scientists have extensive experience in neuroscience, in particular pain, gained from both academic and industry environments. By partnering with Metrion, customers benefit from:

Metrion continues to lead the way in ion channel research. Our NaV1.9 capabilities represent a significant advancement in pain drug discovery. By addressing key challenges associated with NaV1.9, the use of cutting-edge automated patch clamp technology and rodent translational models, Metrion is ready to support your projects to identify new pain therapies.
Beyond NaV1.9, Metrion offers an extensive portfolio of ion channel screening services, including validated assays for the full Comprehensive in vitro Proarrhythmia Assay (CiPA) panel for cardiac safety assessment: hERG, hNav1.5, hCav1.2, hKir 2.1, hKv 4.3/KChIP, hKv LQT1/minK, Late Nav1.5.
This breadth allows customers to consolidate their ion channel research needs with a single provider, streamlining project management and communication.
How advancements in ion channel drug discovery are developing research in the field of pain management
Enhance the predictive accuracy of cardiac safety assessments to ensure safer drug development and reduce the likelihood of late-stage failures due to cardiac toxicity