Academic case study on Nav1.9 as a drug target by Associate Professor David Bulmer (University of Cambridge). Overview of Metrion’s newly developed Nav1.9 screening assays by Metrion CSO, Dr. Eddy Stevens.
The use of brain slice tissue in central nervous system (CNS) drug discovery offers an indispensable platform for understanding the intricate effects of pharmacological compounds in a controlled, yet physiologically relevant, environment. Unlike isolated or cultured brain cells, brain slices maintain the anatomical and synaptic architecture of the brain, providing a more accurate reflection of in vivo conditions. This enables a more integrated view of neuronal activity, allowing for detailed investigations into ion channel function, synaptic transmission, and overall excitatory activity.
By bridging the gap between isolated cell studies and whole-animal models, brain slices play a critical role in identifying and optimising drug candidates for neurological and psychiatric disorders. This approach ultimately increases the likelihood of successful therapeutic outcomes by providing insights that are crucial for the development of effective CNS drugs.
Electrophysiological Recordings: With extensive experience in manual patch-clamp techniques across various brain regions, we provide detailed insights into the electrophysiological properties of neurons.
Experimental Focus: We study both passive and active membrane properties, characterising neuronal currents and exploring synaptic plasticity and transmission in response to pharmacological agents.
Acute Rodent Brain Slices: Electrophysiological analysis performed on freshly prepared rodent brain slices.
Diverse Endpoints: Including intrinsic firing properties, as well as spontaneous and evoked neuronal responses.

Figure 1. Hippocampal spontaneous post-synaptic currents (sPSCs) and current-clamp recordings of action potential firing. A. Representative bright field image of a rodent hippocampus, with the subfields cornu ammonis 1 (CA1) and 3 (CA3), and dentate gyrus (DG) indicated. A representative neuron from CA1 (indicated within the box) is shown with a glass recording electrode attached during an experiment (right panel). B. Representative recordings of spontaneous post-synaptic currents at -70 mV holding potential. The inset illustrates a zoomed-in area of the recording. C. Action potential responses to 1-second pulses of injected current as indicated in the current-clamp protocol (upper panel).
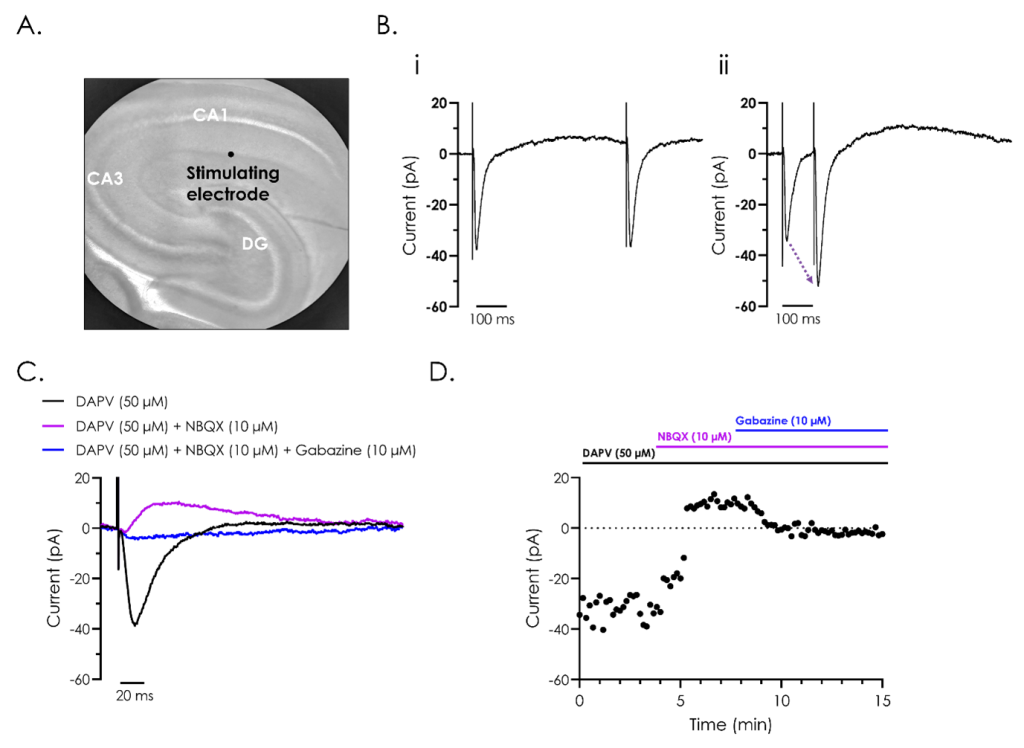
Figure 2. Pharmacological and electrophysiological characterisation of hippocampal evoked post-synaptic currents.
A. Representative bright field image of a rodent hippocampus (left panel), with the subfields cornu ammonis 1 (CA1) and 3 (CA3), and dentate gyrus (DG) indicated. A stimulating electrode was attached close to CA1, as illustrated.
B. A representative recording of evoked post-synaptic currents from hippocampal neurons from CA1, utilising ‘paired-pulse’ stimulation to investigate synaptic plasticity. The effect of reducing the inter-stimulus interval from 500 (i) to 100 (ii) ms illustrates synaptic facilitation, whereby the amplitude of the second evoked current is enhanced as illustrated by the dashed arrow in panel ii.
C. Representative recordings of evoked post-synaptic currents illustrating the different components which make up evoked post-synaptic currents. Recordings were performed after exposure to the NMDA receptor inhibitor D (-)-2-amino-5-phosphonvalerate (DAPV), both alone and in combination with the AMPA receptor inhibitor 2,3-dioxo-6-nitro-7-sulfamoyl-benzo(f)quinoxaline (NBQX), and GABA antagonist, gabazine.
D. A representative current-time plot showing the peak inward and outward currents recorded from the different components of the evoked post-synaptic currents during a 15-minute recording.
Academic case study on Nav1.9 as a drug target by Associate Professor David Bulmer (University of Cambridge). Overview of Metrion’s newly developed Nav1.9 screening assays by Metrion CSO, Dr. Eddy Stevens.
Alex Haworth, Senior Scientist at Metrion, introduces a poster demonstrating Metrion's development of a monoclonal CHO cell line expressing hNav1.9, validated via manual and automated patch clamp techniques.