CiPA logoThe International Council on Harmonization (ICH) S7B and E14 regulatory guidelines were introduced in 2005 to evaluate the proarrhythmic liability of new drugs.
Metrion was an early and active participant in the Comprehensive In vitro Proarrhythmia Assay (CiPA) ion channel high-throughput screening (HTS) sub-team, playing a key role in advancing the initiative. In addition to our involvement with CiPA, we were also an integral member of the Health and Environmental Sciences Institute (HESI) cardiac committee, where we collaborated closely with HESI and other stakeholders in the field to enhance the accuracy and reliability of cardiac safety assessments.
Our expertise was valuable in helping to reduce data variability across multiple screening sites, ensuring that results were consistent and reproducible. Furthermore, we provided crucial validation data to support the development of predictive in silico models, which have become an essential tool in risk assessment for drug-induced arrhythmias.
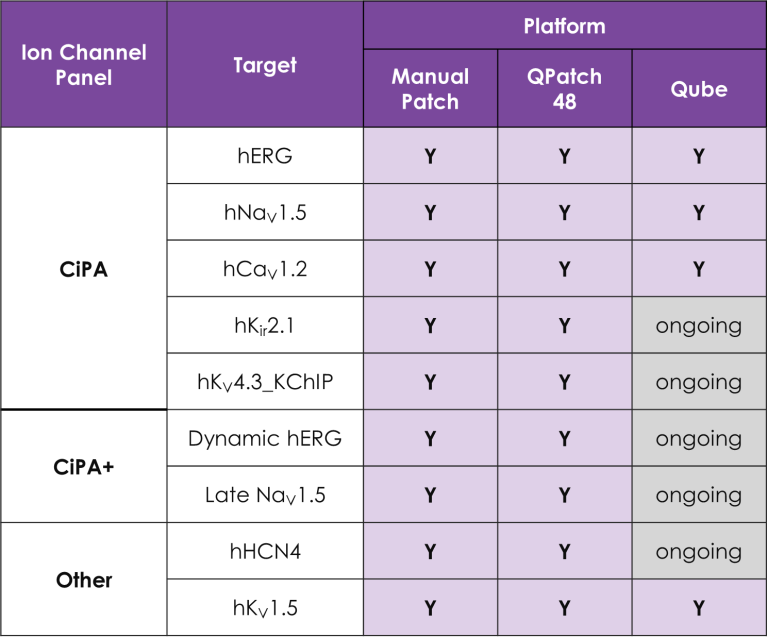
A portfolio of highly validated assays targeting a broad range of human ventricular ion channels, including those that form the full CiPA panel are developed and maintained by Metrion: hERG (human Ether-à-go-go-Related Gene), peak and late Nav1.5 (sodium channel), Cav1.2 (L-type calcium channel), KCNQ1/KCNE1 (potassium channels), Kir2.1 (inward rectifier potassium channel), and Kv4.3 (a component of the delayed rectifier potassium channel) as outlined in Table 1. In addition, we have pioneered the development of a robust dynamic hERG assay, which has been leveraged to provide critical data that contributes to improving the accuracy of the Food and Drug Administration’s (FDA) in silico qNet model, a tool used for predicting cardiac risk in drug development. Beyond the CiPA panel, Metrion offers comprehensive screening services for additional ion channels such as HCN4 and Kv1.5, which play pivotal roles in regulating human heart rate and atrial repolarisation, respectively. Our ability to offer these screening services makes us a valuable partner in advancing cardiac safety and improving the overall efficiency of the drug development process.
Table 1. Assays for a wide range of different human cardiac ion channels.
We provide potency assessments against each cardiac ion channel using single-point or four-point concentration-response assays using:
The potency data derived from these high-fidelity platforms are suitable for use in in silico action potential models, a key component of the CiPA initiative.
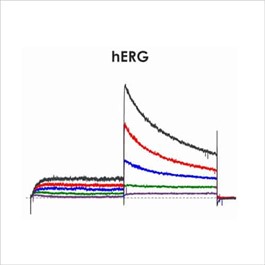
Figure 1a. CiPA ion channel panel – hERG
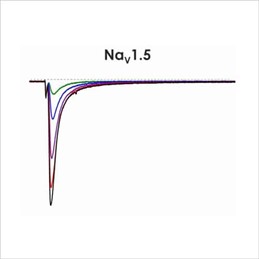
Figure 1b. CiPA ion channel panel – NaV1.5
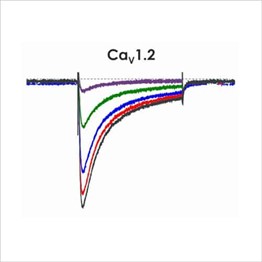
Figure 1c. CiPA ion channel panel – CaV1.2
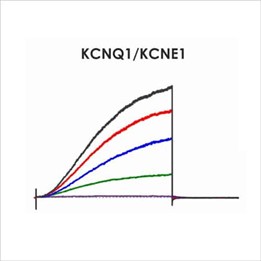
Figure 1d. CiPA ion channel panel – KCNQ1 KCNE1
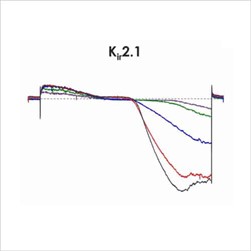
Figure 1e. CiPA ion channel panel – Kir2.1
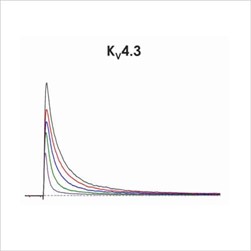
Figure 1f. CiPA ion channel panel – Kv4.3
Cardiac screening assays have been meticulously developed for key ion channels involved in cardiac function, including hERG (human Ether-à-go-go Related Gene), NaV1.5 (sodium channel), and CaV1.2 (L-type calcium channel). These assays are performed using either the Sophion QPatch-48 or Qube, the latter being a state-of-the-art 384-well automated patch-clamp platform, which allows for high-throughput, precise measurements of ion channel activity. The Qube platform provides flexibility in assay configurations, ensuring that various types of screening requirements - ranging from early-stage compound testing to more complex mechanistic investigations - can be effectively addressed. These assays are critical for assessing drug-induced arrhythmic risks, as these ion channels play pivotal roles in regulating the heart's electrical activity. By evaluating compounds against these key channels, we can gather valuable data on their potential to disrupt normal cardiac rhythms, helping to mitigate the risks associated with drug development.
A version of the challenging ‘Milnes’ dynamic hERG voltage protocol suitable for automated patch clamp has been developed and validated by Metron. Allowing for a comprehensive analysis of larger compound numbers in this assay.
The Milnes Assay refers to a specific dynamic voltage-clamp protocol designed to assess the function of the hERG (human Ether-à-go-go Related Gene) potassium channel, which plays a critical role in the repolarisation phase of the cardiac action potential. This protocol was developed by Dr. Robert Milnes and colleagues to better understand how compounds interact with the hERG channel, particularly in the context of assessing their potential to cause arrhythmias, such as Torsades de Pointes, a potentially fatal form of arrhythmia.
The Milnes Assay is particularly useful for evaluating how drugs or compounds may inhibit or modulate the hERG channel under dynamic conditions that more closely mimic physiological behaviour. Unlike traditional methods that use static voltage protocols (which measure channel activity at fixed voltages), the Milnes protocol introduces a more complex dynamic voltage profile, enabling a better simulation of real-world, physiologically relevant conditions. This is critical because it allows researchers to understand how compounds interact with the hERG channel over a range of voltages and time periods, which is especially important in the context of cardiac safety screening.
In the context of automated patch-clamp platforms, like the Qube, the Milnes dynamic protocol allows for more efficient high-throughput screening of large numbers of compounds while maintaining the accuracy required to predict potential risks to the heart's electrical system.
CiPA logoThe International Council on Harmonization (ICH) S7B and E14 regulatory guidelines were introduced in 2005 to evaluate the proarrhythmic liability of new drugs.
In light of the FDA’s changing stance, the Volta assay emerges as a powerful, validated NAM capable of addressing the very safety concerns traditionally assessed in animal studies.