Presented at the SPS meeting in September 2024, Dr Steve Jenkinson presents Metrion’s human induced pluripotent stem cell (hiPSC)-derived cardiomyocyte assay, an advanced tool, providing key advantages, for early cardiac derisking in drug discovery.
High-quality cardiac safety screening services, including GLP hERG and CiPA assays
The cardiac safety screening assays we offer include:
Specialist GLP testing services against hERG using the conventional whole-cell patch-clamp technique.
Generate GLP hERG data to support Investigational New Drug (IND) applications.
Provide an early assessment of potential off-target effects on cardiac ion channels by studying the effect of compounds on the CiPA ion channel panel.
A powerful tool for safer and more efficient drug discovery.
Non-GLP screening services to eliminate cardiac risk liability before lead development.
By working with us you benefit from:
Exceptional ion channel electrophysiology and drug discovery expertise.
A team of experienced cell biologists to create novel cell lines.
High quality, cost-effective compound screening.
Detailed characterisation of lead compounds in a range of high quality assays.
Translational services including confirmation of efficacy in stem cell and other phenotypic models.
Flexible approach that best suits your project and budget.
Rapid turn-around times, reporting and data interpretation by highly experienced ion channel scientists.
Our expertise in GLP hERG screening is demonstrated in the case study GLP hERG Assay Validation Following ICH E14/S7B 2022 Q&A Best Practice Guidelines.
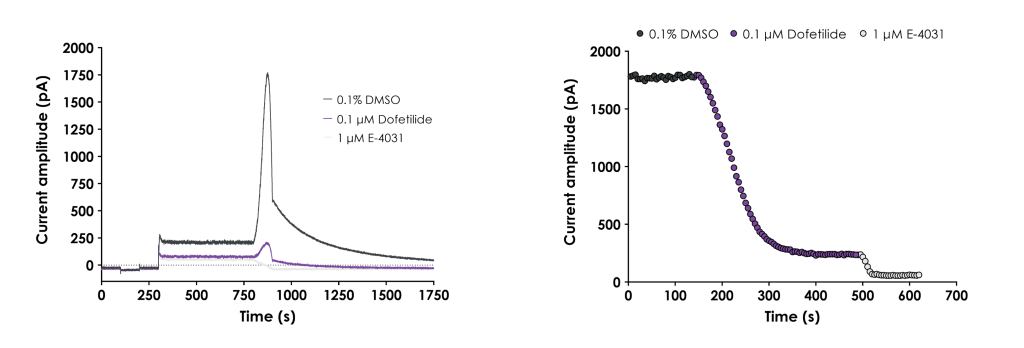
Top: Example traces in vehicle, dofetilide and E-4031. Bottom: Corresponding IT plot. IC50 values generated were within 2-fold of the values reported in the ICH E14/S7B training material. Read more about GLP hERG assay validation.
Download the recording of this webinar to learn how an hiPSC-CM model can help provide clear decision-making data for your project team that can avoid costly issues related to QTc and QRS cardiac liabilities in the clinic.
Recording includes presentations and Q&A:
Derek Leishman (VP Translational and Quantitative Toxicology, Eli Lilly and Company).
Steve Jenkinson (VP Drug Discovery and Safety, Metrion).
Presented at the SPS meeting in September 2024, Dr Steve Jenkinson presents Metrion’s human induced pluripotent stem cell (hiPSC)-derived cardiomyocyte assay, an advanced tool, providing key advantages, for early cardiac derisking in drug discovery.
Jenkinson, Steve Advanced In Vitro Screening of New Drugs for Proarrhythmic Activity, Genetic Engineering News. 2024 44:5, 48-50