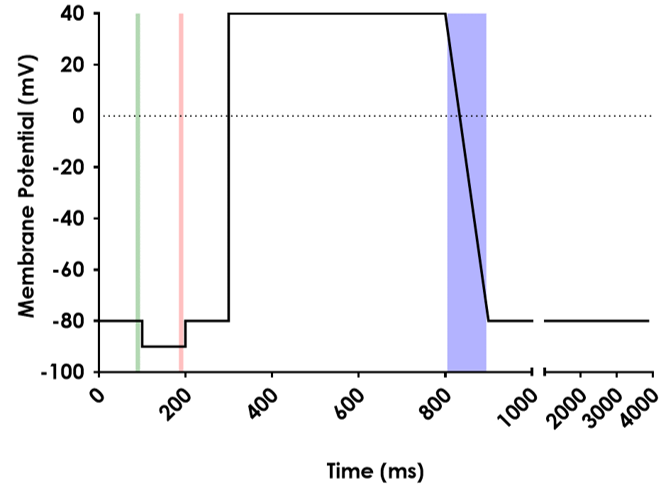
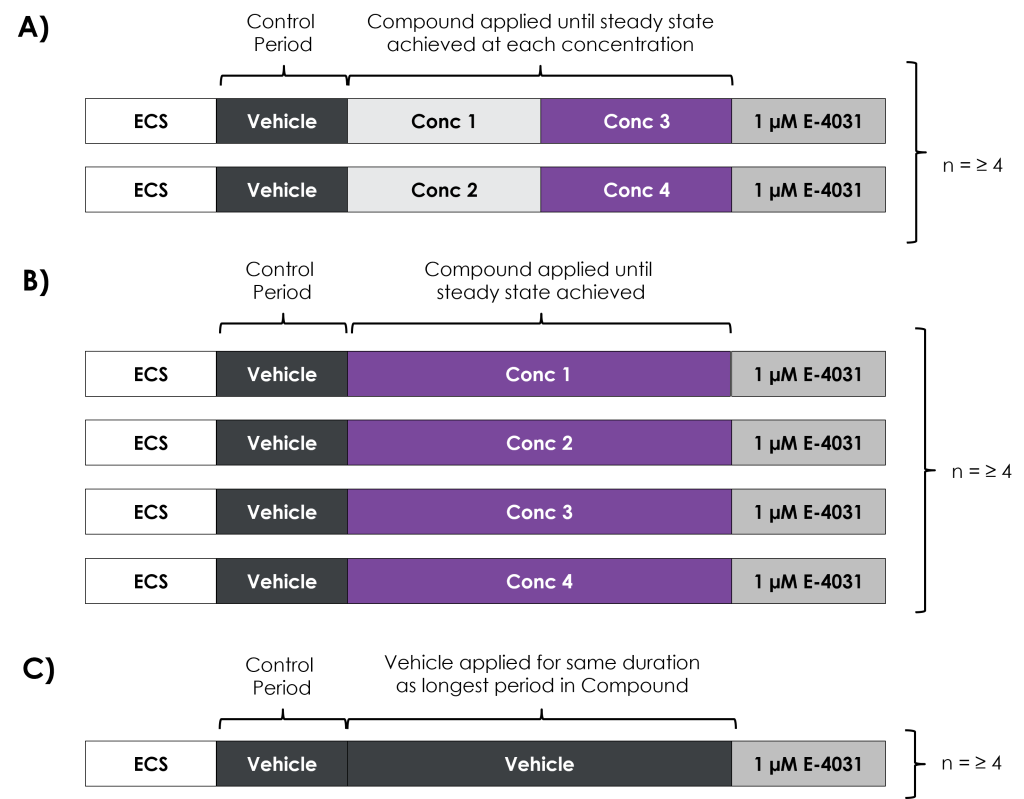
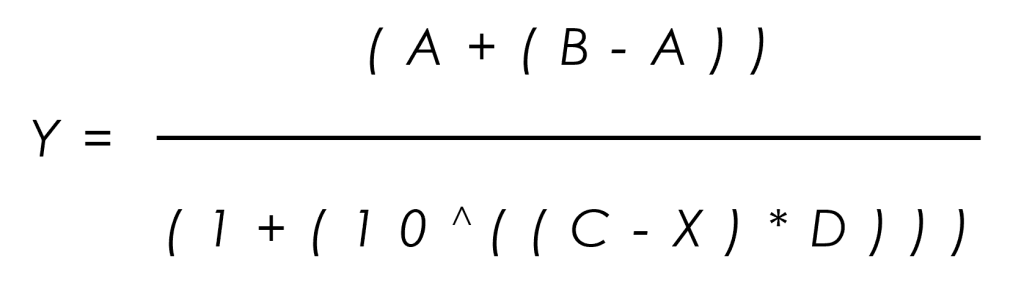The ICH E14/S7B 2022 Q&As provide the best practice guidelines for evaluating the effect of preclinical compounds on the human ether-à-go-go-related gene (hERG) potassium channel1. The guidelines stipulate that in vitro hERG assessments should be performed to Good Laboratory Practice (GLP) compliance. In addition, they provide recommendations on the experimental methods that should be employed, the quality control parameters for analysing the data, as well as the preferred format for reporting the data. This is to ensure data quality, transparency and consistency throughout the industry.
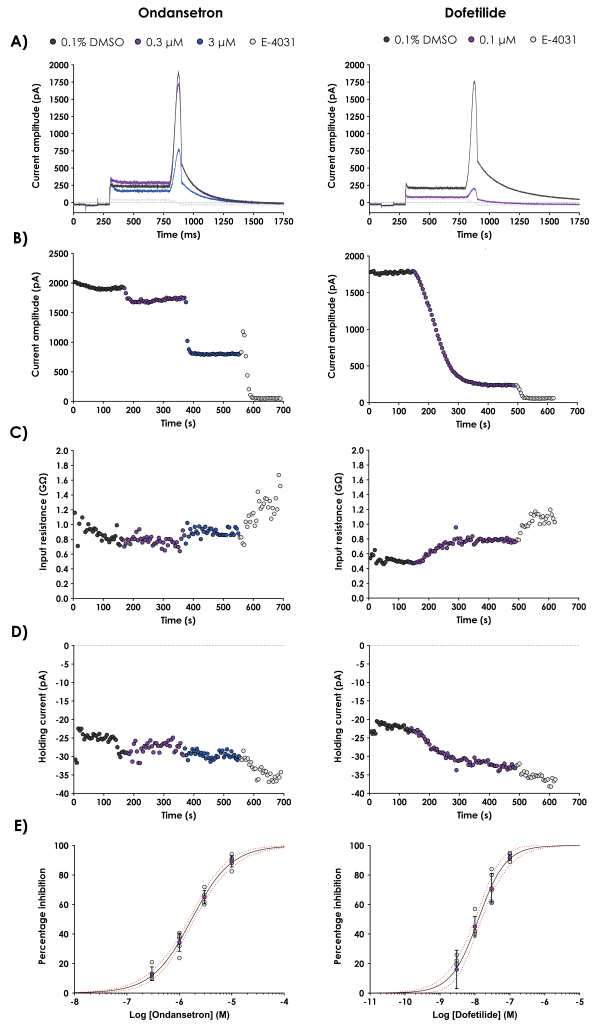
A hERG assay is considered negative if the safety margin calculated for the test article is greater than the established safety margins generated with the Food and Drug Administration’s (FDA’s) positive controls (ondansetron, moxifloxacin and dofetilide) tested to the best practice guidelines. Metrion conducted a GLP compliant study using the conventional manual patch-clamp technique in accordance with the ICH E14/S7B Q&A best practice guidelines to establish in-house IC50 values for ondansetron, moxifloxacin and dofetilide.